Abstract
Purpose
To evaluate the prognostic utility of nodal metabolic parameters derived from FDG PET/CT performed before radiotherapy (prePET) and during the third week of radiotherapy (iPET) in patients with mucosal primary head and neck squamous cell carcinoma (MPHNSCC).
Methods
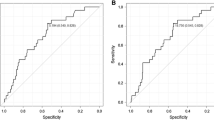
This analysis included 75 patients with newly diagnosed locally advanced node-positive MPHNSCC treated with radical radiotherapy and concurrent systemic therapy who underwent prePET and iPET: N1 11 patients, N2a 38, N2b 12, N2c 9, N3 5. The median follow-up was 28 months (9 – 70 months). The maximum and mean standardized uptake values (SUVmax and SUVmean), metabolic tumour volume (MTV) and total lesional glycolysis (TLG) of the index lymph node (node with the highest TLG) and the combined total lymph nodes, and their percentage reductions on iPET were determined, and the results were correlated with 3-year Kaplan-Meier locoregional, regional and distant metastatic failure-free survival (FFS), disease-free survival (DFS) and overall survival (OS). Optimal cut-off values were derived from receiver operating characteristic curves. Cox regression univariate and multivariate analyses with clinical covariates were performed.
Results
Based on assessment of residual nodal metabolic burden during treatment, the iPET index node SUVmean (optimal cut-off value 2.95 g/ml) and the total node SUVmean (optimal cut-off value 3.25) were the best independent predictors of outcome in the multivariate analysis: index node SUVmean for DFS and OS p = 0.033 and 0.003, respectively, and the total node SUVmean for locoregional FFS, DFS and OS p = 0.028, 0.025 and 0.014, respectively. Based on the assessment of response rates during treatment, a reduction of more than 50 % in the total node TLG was the best biomarker for locoregional and regional FFS, DFS and OS in the multivariate analysis (p = 0.001, 0.016, 0.001 and 0.004, respectively), and reduction in the total node MTV for locoregional FFS, DFS and OS (p = 0.026, 0.003 and 0.014, respectively). There were no significant correlations between oncological outcomes and prePET nodal parameters.
Conclusion
We demonstrated that the index node and total node SUVmean on iPET and a reduction of more than 50 % in MTV and TLG are useful imaging biomarkers, and can potentially identify those patients with MPHNSCC who have a high risk of locoregional metastatic failure and death.



Similar content being viewed by others
References
Marta GN, Silva V, de Andrade Carvalho H, de Arruda FF, Hanna SA, Gadia R, et al. Intensity-modulated radiation therapy for head and neck cancer: systematic review and meta-analysis. Radiother Oncol. 2014;110(1):9–15.
Corry J, Peters LJ, Rischin D. Optimising the therapeutic ratio in head and neck cancer. Lancet Oncol. 2010;11(3):287–291.
Gregoire V, Jeraj R, Lee JA, O’Sullivan B. Radiotherapy for head and neck tumours in 2012 and beyond: conformal, tailored, and adaptive? Lancet Oncol. 2012;13(7):e292–e300.
Chao KS, Ozyigit G, Tran BN, Cengiz M, Dempsey JF, Low DA. Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2003;55(2):312–321.
Gupta T, Master Z, Kannan S, Agarwal JP, Ghsoh-Laskar S, Rangarajan V, et al. Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2011;38(11):2083–2095.
Ceulemans G, Voordeckers M, Farrag A, Verdries D, Storme G, Everaert H. Can 18-FDG-PET during radiotherapy replace post-therapy scanning for detection/demonstration of tumor response in head-and-neck cancer? Int J Radiat Oncol Biol Phys. 2011;81(4):938–942.
Castaldi P, Rufini V, Bussu F, Micciche F, Dinapoli N, Autorino R, et al. Can “early” and “late”18F-FDG PET-CT be used as prognostic factors for the clinical outcome of patients with locally advanced head and neck cancer treated with radio-chemotherapy? Radiother Oncol. 2012;103(1):63–8.
Brun E, Kjellen E, Tennvall J, Ohlsson T, Sandell A, Perfekt R, et al. FDG PET studies during treatment: prediction of therapy outcome in head and neck squamous cell carcinoma. Head Neck. 2002;24(2):127–135.
Hentschel M, Appold S, Schreiber A, Abolmaali N, Abramyuk A, Dorr W, et al. Early FDG PET at 10 or 20 Gy under chemoradiotherapy is prognostic for locoregional control and overall survival in patients with head and neck cancer. Eur J Nucl Med Mol Imaging. 2011;38(7):1203–1211.
Min M, Lin P, Lee MT, Shon IH, Lin M, Forstner D, et al. Prognostic role of metabolic parameters of 18F-FDG PET-CT scan performed during radiation therapy in locally advanced head and neck squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2015;42(13):1984–1994.
Min M, Lin P, Lee M, Shon IH, Lin M, Forstner D, et al. 18F-FDG PET-CT performed before and during radiation therapy of head and neck squamous cell carcinoma: are they independent or complementary to each other? J Med Imaging Radiat Oncol. 2016;60(3):433–440.
Min M, Lin P, Liney G, Lee M, Forstner D, Fowler A, et al. A review of the predictive role of functional imaging in patients with mucosal primary head and neck cancer treated with radiation therapy. J Med Imaging Radiat Oncol. 2016. doi:10.1111/1754-9485.12496.
Lin P, Min M, Lee M, Holloway L, Forstner D, Bray V, et al. Prognostic utility of 18F-FDG PET-CT performed prior to and during primary radiotherapy for nasopharyngeal carcinoma: index node is a useful prognostic imaging biomarker site. Radiother Oncol. 2016;120(1):87–91.
Porceddu SV, Pryor DI, Burmeister E, Burmeister BH, Poulsen MG, Foote MC, et al. Results of a prospective study of positron emission tomography-directed management of residual nodal abnormalities in node‐positive head and neck cancer after definitive radiotherapy with or without systemic therapy. Head Neck. 2011;33(12):1675–1682.
Mehanna H, Wong W-L, McConkey CC, Rahman JK, Robinson M, Hartley AG, et al. PET-CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med. 2016;374(15):1444–1454.
Sjövall J, Bitzén U, Kjellén E, Nilsson P, Wahlberg P, Brun E. Qualitative interpretation of PET scans using a Likert scale to assess neck node response to radiotherapy in head and neck cancer. Eur J Nucl Med Mol Imaging. 2016;43(4):609–616.
Marcus C, Ciarallo A, Tahari AK, Mena E, Koch W, Wahl RL, et al. Head and neck PET/CT: therapy response interpretation criteria (Hopkins criteria) – interreader reliability, accuracy, and survival outcomes. J Nucl Med. 2014;55(9):1411–1416.
Min M, Lin P, Lee M, Ho Shon I, Lin M, Forstner D, et al. Prognostic value of 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography scan carried out during and after radiation therapy for head and neck cancer using visual therapy response interpretation criteria. Clin Oncol. 2016;28(6):393–401.
Chung MK, Jeong HS, Son YI, So YK, Park GY, Choi JY, et al. Metabolic tumor volumes by [18F]-fluorodeoxyglucose PET/CT correlate with occult metastasis in oral squamous cell carcinoma of the tongue. Ann Surg Oncol. 2009;16(11):3111–3117.
La TH, Filion EJ, Turnbull BB, Chu JN, Lee P, Nguyen K, et al. Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;74(5):1335–1341.
Kim G, Kim YS, Han EJ, Yoo Ie R, Song JH, Lee SN, et al. FDG-PET/CT as prognostic factor and surveillance tool for postoperative radiation recurrence in locally advanced head and neck cancer. Radiat Oncol J. 2011;29(4):243–251.
Higgins KA, Hoang JK, Roach MC, Chino J, Yoo DS, Turkington TG, et al. Analysis of pretreatment FDG-PET SUV parameters in head-and-neck cancer: tumor SUVmean has superior prognostic value. Int J Radiat Oncol Biol Phys. 2012;82(2):548–553.
Pak K, Cheon GJ, Kang KW, Chung J-K, Kim EE, Lee DS. Prognostic value of SUVmean in oropharyngeal and hypopharyngeal cancers: comparison with SUVmax and other volumetric parameters of 18F-FDG PET. Clin Nucl Med. 2015;40(1):9–13.
Tang C, Murphy JD, Khong B, La TH, Kong C, Fischbein NJ, et al. Validation that metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;83(5):1514–1520.
Pak K, Cheon GJ, Nam HY, Kim SJ, Kang KW, Chung JK, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med. 2014;55(6):884–890.
Van de Wiele C, Kruse V, Smeets P, Sathekge M, Maes A. Predictive and prognostic value of metabolic tumour volume and total lesion glycolysis in solid tumours. Eur J Nucl Med Mol Imaging. 2013;40(2):290–301.
Busk M, Horsman MR, Kristjansen PE, van der Kogel AJ, Bussink J, Overgaard J. Aerobic glycolysis in cancers: implications for the usability of oxygen‐responsive genes and fluorodeoxyglucose‐PET as markers of tissue hypoxia. Int J Cancer. 2008;122(12):2726–2734.
Grégoire V, Langendijk JA, Nuyts S. Advances in radiotherapy for head and neck cancer. J Clin Oncol. 2015;33(29):3277–3284.
Jeong J, Setton JS, Lee NY, Oh JH, Deasy JO. Estimate of the impact of FDG-avidity on the dose required for head and neck radiotherapy local control. Radiother Oncol. 2014;111(3):340–347.
Nyflot MJ, Harari PM, Yip S, Perlman SB, Jeraj R. Correlation of PET images of metabolism, proliferation and hypoxia to characterize tumor phenotype in patients with cancer of the oropharynx. Radiother Oncol. 2012;105(1):36–40.
Dierckx RA, Van de Wiele C. FDG uptake, a surrogate of tumour hypoxia? Eur J Nucl Med Mol Imaging. 2008;35(8):1544–1549.
Busk M, Horsman MR, Jakobsen S, Bussink J, Van der Kogel A, Overgaard J. Cellular uptake of PET tracers of glucose metabolism and hypoxia and their linkage. Eur J Nucl Med Mol Imaging. 2008;35(12):2294–2303.
van Baardwijk A, Dooms C, van Suylen RJ, Verbeken E, Hochstenbag M, Dehing-Oberije C, et al. The maximum uptake of 18F-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1α and GLUT-1 in non-small cell lung cancer. Eur J Cancer. 2007;43(9):1392–1398.
Han MW, Lee HJ, Cho KJ, Kim JS, Roh JL, Choi SH, et al. Role of FDG‐PET as a biological marker for predicting the hypoxic status of tongue cancer. Head Neck. 2012;34(10):1395–1402.
Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Intern Med. 2013;4(2):627–635.
Hilsenbeck SG, Clark GM, McGuire WL. Why do so many prognostic factors fail to pan out? Breast Cancer Res Treat. 1992;22(3):197–206.
Brown E, Owen R, Harden F, Mengersen K, Oestreich K, Houghton W, et al. Predicting the need for adaptive radiotherapy in head and neck cancer. Radiother Oncol. 2015;116(1):57–63.
Brouwer CL, Steenbakkers RJ, Langendijk JA, Sijtsema NM. Identifying patients who may benefit from adaptive radiotherapy: does the literature on anatomic and dosimetric changes in head and neck organs at risk during radiotherapy provide information to help? Radiother Oncol. 2015;115(3):285–294.
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35.
Min M, Lee MT, Lin P, Holloway L, Wijesekera D, Gooneratne D, et al. Assessment of serial multi-parametric functional MRI (diffusion-weighted imaging and R 2*) with 18F-FDG-PET in patients with head and neck cancer treated with radiation therapy. Br J Radiol. 2016;89(1058), 20150530.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflicts of interest
None.
Ethical approval
This study was approved by the local research ethics committee (Sydney South West Area Health Service Human Research Ethics Committee). For this type of study, formal consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 30 kb)
Rights and permissions
About this article
Cite this article
Lin, P., Min, M., Lee, M. et al. Nodal parameters of FDG PET/CT performed during radiotherapy for locally advanced mucosal primary head and neck squamous cell carcinoma can predict treatment outcomes: SUVmean and response rate are useful imaging biomarkers. Eur J Nucl Med Mol Imaging 44, 801–811 (2017). https://doi.org/10.1007/s00259-016-3584-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3584-1