Abstract
Purpose
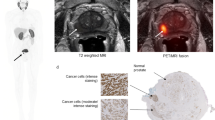
[18F]Fluciclovine PET imaging shows promise for the assessment of prostate cancer. The purpose of this PET/MRI study is to optimise the PET imaging protocol for detection and characterisation of primary prostate cancer, by quantitative evaluation of the dynamic uptake of [18F]Fluciclovine in cancerous and benign tissue.
Methods
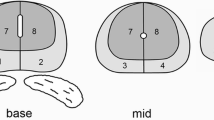
Patients diagnosed with high-risk primary prostate cancer underwent an integrated [18F]Fluciclovine PET/MRI exam before robot-assisted radical prostatectomy with extended pelvic lymph node dissection. Volumes-of-interest (VOIs) of selected organs (prostate, bladder, blood pool) and sub-glandular prostate structures (tumour, benign prostatic hyperplasia (BPH), inflammation, healthy tissue) were delineated on T2-weighted MR images, using whole-mount histology samples as a reference. Three candidate windows for optimal PET imaging were identified based on the dynamic curves of the mean and maximum standardised uptake value (SUVmean and SUVmax, respectively). The statistical significance of differences in SUV between VOIs were analysed using Wilcoxon rank sum tests (p<0.05, adjusted for multiple testing).
Results
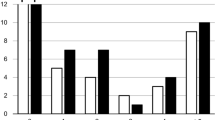
Twenty-eight (28) patients [median (range) age: 66 (55-72) years] were included. An early (W1: 5-10 minutes post-injection) and two late candidate windows (W2: 18-23; W3: 33-38 minutes post-injection) were selected. Late compared with early imaging was better able to distinguish between malignant and benign tissue [W3, SUVmean: tumour vs. BPH 2.5 vs. 2.0 (p<0.001), tumour vs. inflammation 2.5 vs. 1.7 (p<0.001), tumour vs. healthy tissue 2.5 vs. 2.0 (p<0.001); W1, SUVmean: tumour vs. BPH 3.1 vs. 3.1 (p=0.771), tumour vs inflammation 3.1 vs. 2.2 (p=0.021), tumour vs. healthy tissue 3.1 vs. 2.5 (p<0.001)] as well as between high-grade and low/intermediate-grade tumours (W3, SUVmean: 2.6 vs. 2.1 (p=0.040); W1, SUVmean: 3.1 vs. 2.8 (p=0.173)). These differences were relevant to the peripheral zone, but not the central gland.
Conclusion
Late-window [18F]Fluciclovine PET imaging shows promise for distinguishing between prostate tumours and benign tissue and for assessment of tumour aggressiveness.




Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–37.
Barentsz JO, Weinreb JC, Verma S, Thoeny HC, Tempany CM, Shtern F, et al. Synopsis of the PI-RADS v2 guidelines for multiparametric prostate magnetic resonance imaging and recommendations for use. Eur Urol. 2016;69:41–9.
Futterer JJ, Briganti A, De Visschere P, Emberton M, Giannarini G, Kirkham A, et al. Can clinically significant prostate cancer Be detected with multiparametric magnetic resonance imaging? a systematic review of the literature. Eur Urol. 2015;68:1045–53.
Kobus T, Vos PC, Hambrock T, De Rooij M, de Kaa CA H-V, Barentsz JO, et al. Prostate cancer aggressiveness: in vivo assessment of MR spectroscopy and diffusion-weighted imaging at 3 T. Radiology. 2012;265:457–67.
Hovels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63:387–95.
Schuster DM, Votaw JR, Nieh PT, Yu W, Nye JA, Master V, et al. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med. 2007;48:56–63.
Schuster DM, Nieh PT, Jani AB, Amzat R, Bowman FD, Halkar RK, et al. Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol. 2014;191:1446–53.
Nanni C, Zanoni L, Pultrone C, Schiavina R, Brunocilla E, Lodi F et al. F-FACBC (anti1-amino-3-F-fluorocyclobutane-1-carboxylic acid) versus C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging. 2016.
Schuster DM, Taleghani PA, Nieh PT, Master VA, Amzat R, Savir-Baruch B, et al. Characterization of primary prostate carcinoma by anti-1-amino-2-[(18)F] -fluorocyclobutane-1-carboxylic acid (anti-3-[(18)F] FACBC) uptake. Am J Nucl Med Mol Imaging. 2013;3:85–96.
Turkbey B, Mena E, Shih J, Pinto PA, Merino MJ, Lindenberg ML, et al. Localized prostate cancer detection with 18F FACBC PET/CT: comparison with MR imaging and histopathologic analysis. Radiology. 2014;270:849–56.
Schuster DM, Nanni C, Fanti S, Oka S, Okudaira H, Inoue Y, et al. Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid: physiologic uptake patterns, incidental findings, and variants that may simulate disease. J Nucl Med. 2014;55:1986–92.
Sorensen J, Owenius R, Lax M, Johansson S. Regional distribution and kinetics of [18F]fluciclovine (anti-[18F]FACBC), a tracer of amino acid transport, in subjects with primary prostate cancer. Eur J Nucl Med Mol Imaging. 2013;40:394–402.
Epstein JI. An update of the Gleason grading system. J Urol. 2010;183:433–40.
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging. 2012;30:1323–41.
Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17:205–16.
Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, et al. A contemporary prostate cancer grading system: a validated alternative to the gleason score. Eur Urol. 2016;69:428–35.
Klein S, Staring M, Murphy K, Viergever MA. Pluim JP elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2010;29:196–205.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol. 1995;57:289–300.
Acknowledgments
The authors acknowledge Professor Frode Willoch, Institute of Basic Medical Sciences, University of Oslo, for his support with [18F]Fluciclovine PET imaging.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by The Norwegian Cancer Society (grant number 100792).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Elschot, M., Selnæs, K.M., Sandsmark, E. et al. A PET/MRI study towards finding the optimal [18F]Fluciclovine PET protocol for detection and characterisation of primary prostate cancer. Eur J Nucl Med Mol Imaging 44, 695–703 (2017). https://doi.org/10.1007/s00259-016-3562-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3562-7