Abstract
Purpose
Metabolic activity and hypoxia are both important factors characterizing tumor aggressiveness. Here, we used F-18 fluoromisonidazole (FMISO) and F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) to define metabolically active hypoxic volume, and investigate its clinical significance in relation to progression free survival (PFS) and overall survival (OS) in glioblastoma patients.
Experimental Design
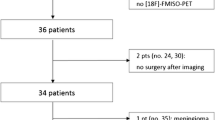
Glioblastoma patients (n = 32) underwent FMISO PET, FDG PET, and magnetic resonance imaging (MRI) before surgical intervention. FDG and FMISO PET images were coregistered with gadolinium-enhanced T1-weighted MR images. Volume of interest (VOI) of gross tumor volume (GTV) was manually created to enclose the entire gadolinium-positive areas. The FMISO tumor-to-normal region ratio (TNR) and FDG TNR were calculated in a voxel-by-voxel manner. For calculating TNR, standardized uptake value (SUV) was divided by averaged SUV of normal references. Contralateral frontal and parietal cortices were used as the reference region for FDG, whereas the cerebellar cortex was used as the reference region for FMISO. FDG-positive was defined as the FDG TNR ≥1.0, and FMISO-positive was defined as FMISO TNR ≥1.3. Hypoxia volume (HV) was defined as the volume of FMISO-positive and metabolic tumor volume in hypoxia (hMTV) was the volume of FMISO/FDG double-positive. The total lesion glycolysis in hypoxia (hTLG) was hMTV × FDG SUVmean. The extent of resection (EOR) involving cytoreduction surgery was volumetric change based on planimetry methods using MRI. These factors were tested for correlation with patient prognosis.
Results
All tumor lesions were FMISO-positive and FDG-positive. Univariate analysis indicated that hMTV, hTLG, and EOR were significantly correlated with PFS (p = 0.007, p = 0.04, and p = 0.01, respectively) and that hMTV, hTLG, and EOR were also significantly correlated with OS (p = 0.0028, p = 0.037, and p = 0.014, respectively). In contrast, none of FDG TNR, FMISO TNR, GTV, HV, patients’ age, or Karnofsky performance scale (KPS) was significantly correlated with PSF or OS. The hMTV and hTLG were found to be independent factors affecting PFS and OS on multivariate analysis.
Conclusions
We introduced hMTV and hTLG using FDG and FMISO PET to define metabolically active hypoxic volume. Univariate and multivariate analyses demonstrated that both hMTV and hTLG are significant predictors for PFS and OS in glioblastoma patients.


Similar content being viewed by others
References
Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi:10.1007/s00401-007-0243-4.
Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–50. doi:10.1001/jama.2013.280319.
Flynn JR, Wang L, Gillespie DL, et al. Hypoxia-regulated protein expression, patient characteristics, and preoperative imaging as predictors of survival in adults with glioblastoma multiforme. Cancer. 2008;113(5):1032–42. doi:10.1002/cncr.23678.
Oliver L, Olivier C, Marhuenda FB, Campone M, Vallette FM. Hypoxia and the malignant glioma microenvironment: regulation and implications for therapy. Curr Mol Pharmacol. 2009;2(3):263–84.
Hirata K, Terasaka S, Shiga T, Hattori N, Magota K, Kobayashi H, et al. 18F-Fluoromisonidazole positron emission tomography may differentiate glioblastoma multiforme from less malignant gliomas. Eur J Nucl Med Mol Imaging. 2012;39(5):760–70. doi:10.1007/s00259-011-2037-0.
Evans SM, Judy KD, Dunphy I, et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res : Off J Am Assoc Cancer Res. 2004;10(24):8177–84. doi:10.1158/1078-0432.CCR-04-1081.
Lally BE, Rockwell S, Fischer DB, Collingridge DR, Piepmeier JM, Knisely JP. The interactions of polarographic measurements of oxygen tension and histological grade in human glioma. Cancer J. 2006;12(6):461–6.
Toyonaga T, Hirata K, Yamaguchi S, Hatanaka KC, Yuzawa S, Manabe O, et al. 18F-fluoromisonidazole positron emission tomography can predict pathological necrosis of brain tumors. Eur J Nucl Med Mol Imaging. 2016. doi:10.1007/s00259-016-3320-x.
Bell C, Dowson N, Fay M, Thomas P, Puttick S, Gal Y, et al. Hypoxia imaging in gliomas with 18F-fluoromisonidazole PET: toward clinical translation. Semin Nucl Med. 2015;45(2):136–50. doi:10.1053/j.semnuclmed.2014.10.001.
Jensen RL. Brain tumor hypoxia: tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a therapeutic target. J Neuro-Oncol. 2009;92(3):317–35. doi:10.1007/s11060-009-9827-2.
Spence AM, Muzi M, Swanson KR, O’Sullivan F, Rockhill JK, Rajendran JG, et al. Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clin Cancer Res: Off J Am Assoc Cancer Res. 2008;14(9):2623–30. doi:10.1158/1078-0432.CCR-07-4995.
Swanson KR, Chakraborty G, Wang CH, Rockne R, Harpold HL, Muzi M, et al. Complementary but distinct roles for MRI and 18F-fluoromisonidazole PET in the assessment of human glioblastomas. J Nucl Med: Off Publ Soc Nucl Med. 2009;50(1):36–44. doi:10.2967/jnumed.108.055467.
Szeto MD, Chakraborty G, Hadley J, et al. Quantitative metrics of net proliferation and invasion link biological aggressiveness assessed by MRI with hypoxia assessed by FMISO-PET in newly diagnosed glioblastomas. Cancer Res. 2009;69(10):4502–9. doi:10.1158/0008-5472.CAN-08-3884.
Kawai N, Lin W, Cao WD, Ogawa D, Miyake K, Haba R, et al. Correlation between 18F-fluoromisonidazole PET and expression of HIF-1alpha and VEGF in newly diagnosed and recurrent malignant gliomas. Eur J Nucl Med Mol Imaging. 2014;41(10):1870–8. doi:10.1007/s00259-014-2776-9.
Colavolpe C, Metellus P, Mancini J, et al. Independent prognostic value of pre-treatment 18-FDG-PET in high-grade gliomas. J Neuro-Oncol. 2012;107(3):527–35. doi:10.1007/s11060-011-0771-6.
Spence AM, Muzi M, Graham MM, O’Sullivan F, Link JM, Lewellen TK, et al. 2-[18F]Fluoro-2-deoxyglucose and glucose uptake in malignant gliomas before and after radiotherapy: correlation with outcome. Clin Cancer Res: Off J Am Assoc Cancer Res. 2002;8(4):971–9.
Pardo FS, Aronen HJ, Fitzek M, et al. Correlation of FDG-PET interpretation with survival in a cohort of glioma patients. Anticancer Res. 2004;24(4):2359–65.
Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapp. 1994;2(4):189–210. doi:10.1002/hbm.460020402.
Bruehlmeier M, Roelcke U, Schubiger PA, Ametamey SM. Assessment of hypoxia and perfusion in human brain tumors using PET with 18F-fluoromisonidazole and 15O-H2O. J Nucl Med: Off Publ Soc Nucl Med. 2004;45(11):1851–9.
Yamaguchi S, Kobayashi H, Terasaka S, et al. The impact of extent of resection and histological subtype on the outcome of adult patients with high-grade gliomas. Jpn J Clin Oncol. 2012;42(4):270–7. doi:10.1093/jjco/hys016.
Yamaguchi S, Kobayashi H, Hirata K, Shiga T, Tanaka S, Murata J, et al. Detection of histological anaplasia in gliomas with oligodendroglial components using positron emission tomography with 18F-FDG and 11C-methionine: report of two cases. J Neuro-Oncol. 2011;101(2):335–41. doi:10.1007/s11060-010-0262-1.
Kobayashi K, Hirata K, Yamaguchi S, Manabe O, Terasaka S, Kobayashi H, et al. Prognostic value of volume-based measurements on 11C-methionine PET in glioma patients. Eur J Nucl Med Mol Imaging. 2015;42(7):1071–80. doi:10.1007/s00259-015-3046-1.
Yoshikawa K, Kajiwara K, Morioka J, et al. Improvement of functional outcome after radical surgery in glioblastoma patients: the efficacy of a navigation-guided fence-post procedure and neurophysiological monitoring. J Neuro-Oncol. 2006;78(1):91–7. doi:10.1007/s11060-005-9064-2.
Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using "optimal" cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86(11):829–35.
Therneau T. A package for survival analysis in S. version 2.38. 2015. http://CRAN.R-project.org/package=survival. Accessed 25 Sept 2016.
Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. doi:10.3171/2011.2.JNS10998.
Pope WB, Sayre J, Perlina A, Villablanca JP, Mischel PS, Cloughesy TF. MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am J Neuroradiol. 2005;26(10):2466–74.
Keles GE, Anderson B, Berger MS. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol. 1999;52(4):371–9.
Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–64. doi:10.1227/01.neu.0000318159.21731.cf. discussion 264-6.
Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–8. doi:10.3171/jns.2001.95.2.0190.
Hardesty DA, Sanai N. The value of glioma extent of resection in the modern neurosurgical era. Front Neurol. 2012;3:140. doi:10.3389/fneur.2012.00140.
Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE. Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol : Off J Am Soc Clin Oncol. 2014;32(8):774–82. doi:10.1200/JCO.2013.51.8886.
Gao JL, Chen YG. Natural Compounds Regulate Glycolysis in Hypoxic Tumor Microenvironment. Biomed Res Int. 2015;2015:354143. doi:10.1155/2015/354143.
Pak K, Cheon GJ, Nam HY, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med : Off Publ Soc Nucl Med. 2014;55(6):884–90. doi:10.2967/jnumed.113.133801.
Terakawa Y, Tsuyuguchi N, Iwai Y, et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med : Off Publ Soc Nucl Med. 2008;49(5):694–9. doi:10.2967/jnumed.107.048082.
Suzuki H, Nishio M, Nakanishi H, Hanai N, Hirakawa H, Kodaira T, et al. Impact of total lesion glycolysis measured by 18F-FDG-PET/CT on overall survival and distant metastasis in hypopharyngeal cancer. Oncol Lett. 2016;12(2):1493–500. doi:10.3892/ol.2016.4765.
Abd El-Hafez YG, Moustafa HM, Khalil HF, Liao CT, Yen TC. Total lesion glycolysis: a possible new prognostic parameter in oral cavity squamous cell carcinoma. Oral Oncol. 2013;49(3):261–8. doi:10.1016/j.oraloncology.2012.09.005.
Lim R, Eaton A, Lee NY, Setton J, Ohri N, Rao S, et al. 18F-FDG PET/CT metabolic tumor volume and total lesion glycolysis predict outcome in oropharyngeal squamous cell carcinoma. J Nucl Med : Off Publ Soc Nucl Med. 2012;53(10):1506–13. doi:10.2967/jnumed.111.101402.
Moon SH, Choi JY, Lee HJ, Son YI, Baek CH, Ahn YC, et al. Prognostic value of 18F-FDG PET/CT in patients with squamous cell carcinoma of the tonsil: comparisons of volume-based metabolic parameters. Head Neck. 2013;35(1):15–22. doi:10.1002/hed.22904.
Picchio M, Kirienko M, Mapelli P, Dell’Oca I, Villa E, Gallivanone F, et al. Predictive value of pre-therapy 18F-FDG PET/CT for the outcome of 18F-FDG PET-guided radiotherapy in patients with head and neck cancer. Eur J Nucl Med Mol Imaging. 2014;41(1):21–31. doi:10.1007/s00259-013-2528-2.
Meijer TW, Kaanders JH, Span PN, Bussink J. Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clin Cancer Res : Off J Am Assoc Cancer Res. 2012;18(20):5585–94. doi:10.1158/1078-0432.CCR-12-0858.
Groussard C, Morel I, Chevanne M, Monnier M, Cillard J, Delamarche A. Free radical scavenging and antioxidant effects of lactate ion: an in vitro study. J Appl Physiol. 2000;89(1):169–75.
Sundar SJ, Hsieh JK, Manjila S, Lathia JD, Sloan A. The role of cancer stem cells in glioblastoma. Neurosurg Focus. 2014;37(6), E6. doi:10.3171/2014.9.FOCUS14494.
Yasuda K, Onimaru R, Okamoto S, Shiga T, Katoh N, Tsuchiya K, et al. [18F]fluoromisonidazole and a new PET system with semiconductor detectors and a depth of interaction system for intensity modulated radiation therapy for nasopharyngeal cancer. Int J Radiat Oncol Biol Phys. 2013;85(1):142–7. doi:10.1016/j.ijrobp.2012.03.029.
Hatano T, Zhao S, Zhao Y, et al. Biological characteristics of intratumoral [F-18]fluoromisonidazole distribution in a rodent model of glioma. Int J Oncol. 2013;42(3):823–30. doi:10.3892/ijo.2013.1781.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflicts of interest
Financial disclosure
This work was supported in part by the Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program, Ministry of Education, Culture, Sports, Science and Technology, Japan, from the SNMMI Wagner–Torizuka Fellowship from 2013 to 2015 (to Kenji Hirata), by the Hokkaido University HIROKO’s Fund for Academic Exchange from 2012 to 2014 (to Kenji Hirata), and by a research grant from the Japan Radiological Society from Bayer from April 2015 to March 2016 (to Kenji Hirata). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 645 kb)
Rights and permissions
About this article
Cite this article
Toyonaga, T., Yamaguchi, S., Hirata, K. et al. Hypoxic glucose metabolism in glioblastoma as a potential prognostic factor. Eur J Nucl Med Mol Imaging 44, 611–619 (2017). https://doi.org/10.1007/s00259-016-3541-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3541-z