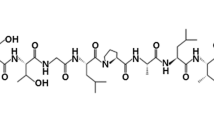
Abstract
Many fungal diseases remain poorly addressed by public health authorities, despite posing a substantial threat to humans, animals, and plants. More worryingly, few classes of anti-fungals have been developed to combat fungal infections thus far. These medications also have certain drawbacks in terms of toxicity, spectrum of activity, and pharmacokinetic properties. Hence, there is a dire need for discovery of novel anti-fungal agents. Melittin, the main constituent in the venom of European honeybee Apis mellifera, has attracted considerable attention among researchers owing to its potential therapeutic applications. To our knowledge, there has been no review pertinent to anti-fungal properties of melittin, prompting us to synopsize the results of experimental investigations with a special emphasis upon underlying mechanisms. In this respect, melittin inhibits a broad spectrum of fungal genera including Aspergillus, Botrytis, Candida, Colletotrichum, Fusarium, Malassezia, Neurospora, Penicillium, Saccharomyces, Trichoderma, Trichophyton, and Trichosporon. Melittin hinders fungal growth by several mechanisms such as membrane permeabilization, apoptosis induction by reactive oxygen species-mediated mitochondria/caspase-dependent pathway, inhibition of (1,3)-β-d-glucan synthase, and alterations in fungal gene expression. Overall, melittin will definitely open up new avenues for various biomedical applications, from medicine to agriculture.
Keypoints
• Venom-derived peptides have potential for development of anti-microbial agents.
• Many fungal pathogens are susceptible to melittin at micromolar concentrations.
• Melittin possesses multi-target mechanism of action against fungal cells.

Similar content being viewed by others
References
Al-Ani I, Zimmermann S, Reichling J, Wink M (2015) Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 22(2):245–255. https://doi.org/10.1016/j.phymed.2014.11.019
Almeida F, Rodrigues ML, Coelho C (2019) The still underestimated problem of fungal diseases worldwide. Front Microbiol 10:214. https://doi.org/10.3389/fmicb.2019.00214
Andrä J, Leippe M (1999) Candidacidal activity of shortened synthetic analogs of amoebapores and NK-lysin. Med Microbiol Immunol 188(3):117–124. https://doi.org/10.1007/s004300050113
Andrä J, Berninghausen O, Leippe M (2001) Cecropins, antibacterial peptides from insects and mammals, are potently fungicidal against Candida albicans. Med Microbiol Immunol 189(3):169–173. https://doi.org/10.1007/s430-001-8025-x
Ayofemi Olalekan Adeyeye S (2020) Aflatoxigenic fungi and mycotoxins in food: a review. Crit Rev Food Sci Nutr 60(5):709–721. https://doi.org/10.1080/10408398.2018
Benedict K, Chiller TM, Mody RK (2016) Invasive fungal infections acquired from contaminated food or nutritional supplements: a review of the literature. Foodborne Pathog Dis 13(7):343–349. https://doi.org/10.1089/fpd.2015.2108
Bongomin F, Gago S, Oladele RO, Denning DW (2017) Global and multi-national prevalence of fungal diseases – estimate precision. J Fungi (Basel) 3(4):57. https://doi.org/10.3390/jof3040057
Cannon PF, Damm U, Johnston PR, Weir BS (2012) Colletotrichum – current status and future directions. Stud Mycol 73(1):181–213. https://doi.org/10.3114/sim0014
Chen L, Wang Z, Liu L, Qu S, Mao Y, Peng X, Li YX, Tian J (2019) Cinnamaldehyde inhibits Candida albicans growth by causing apoptosis and its treatment on vulvovaginal candidiasis and oropharyngeal candidiasis. Appl Microbiol Biotechnol 103(21-22):9037–9055. https://doi.org/10.1007/s00253-019-10119-3
Choi H, Lee DG (2014) Antifungal activity and pore-forming mechanism of astacidin 1 against Candida albicans. Biochimie 105:58–63. https://doi.org/10.1016/j.biochi.2014.06.014
Choi H, Hwang JS, Kim H, Lee DG (2013a) Antifungal effect of CopA3 monomer peptide via membrane-active mechanism and stability to proteolysis of enantiomeric D-CopA3. Biochem Biophys Res Commun 440(1):94–98. https://doi.org/10.1016/j.bbrc.2013.09.021
Choi H, Hwang JS, Lee DG (2013b) Antifungal effect and pore-forming action of lactoferricin B like peptide derived from centipede Scolopendra subspinipes mutilans. Biochim Biophys Acta 1828(11):2745–2750. https://doi.org/10.1016/j.bbamem.2013.07.021
Clark GC, Casewell NR, Elliott CT, Harvey AL, Jamieson AG, Strong PN, Turner AD (2019) Friends or foes? Emerging impacts of biological toxins. Trends Biochem Sci 44(4):365–379. https://doi.org/10.1016/j.tibs.2018.12.004
Conrad KA, Rodriguez R, Salcedo EC, Rauceo JM (2018) The Candida albicans stress response gene stomatin-like protein 3 is implicated in ROS-induced apoptotic-like death of yeast phase cells. PLoS One 13(2):e0192250. https://doi.org/10.1371/journal.pone.0192250
de Almeida Júnior JN, Hennequin C (2016) Invasive Trichosporon infection: a systematic review on a re-emerging fungal pathogen. Front Microbiol 7:1629. https://doi.org/10.3389/fmicb.2016.01629
de Oliveira Santos GC, Vasconcelos CC, Lopes AJO, de Sousa Cartágenes MDS, Filho AKDB, do Nascimento FRF, Ramos RM, Pires ERRB, de Andrade MS, Rocha FMG, de Andrade Monteiro C (2018) Candida infections and therapeutic strategies: mechanisms of action for traditional and alternative agents. Front Microbiol 9:1351. https://doi.org/10.3389/fmicb.2018.01351
DeGrado WF, Musso GF, Lieber M, Kaiser ET, Kézdy FJ (1982) Kinetics and mechanism of hemolysis induced by melittin and by a synthetic melittin analogue. Biophys J 1:329–338. https://doi.org/10.1016/S0006-3495(82)84681-X
Do N, Weindl G, Grohmann L, Salwiczek M, Koksch B, Korting HC, Schäfer-Korting M (2014) Cationic membrane-active peptides - anticancer and antifungal activity as well as penetration into human skin. Exp Dermatol 23(5):326–331. https://doi.org/10.1111/exd.12384
Dorman LC, Markley LD (1971) Solid phase synthesis and antibacterial activity of N-terminal sequences of melittin. J Med Chem 14(1):5–9. https://doi.org/10.1021/jm00283a003
Fennell JF, Shipman WH, Cole LJ (1967) Antibacterial action of a bee venom fraction (melittin) against a penicillin-resistant Staphylococcus and other microorganisms. USNRDL-TR-67-101. Res Dev Tech Rep 5:1–13
Giorgi C, Baldassari F, Bononi A, Bonora M, De Marchi E, Marchi S, Missiroli S, Patergnani S, Rimessi A, Suski JM, Wieckowski MR, Pinton P (2012) Mitochondrial Ca2+ and apoptosis. Cell Calcium 52(1):36–43. https://doi.org/10.1016/j.ceca.2012.02.008
Gopal R, Na H, Seo CH, Park Y (2012) Antifungal activity of (KW)n or (RW)n peptide against Fusarium solani and Fusarium oxysporum. Int J Mol Sci 13(11):15042–15053. https://doi.org/10.3390/ijms131115042
Habermann E (1972) Bee and wasp venoms. Science 177(4046):314–322. https://doi.org/10.1126/science.177.4046.314
Hallstrom TC, Lambert L, Schorling S, Balzi E, Goffeau A, Moye-Rowley WS (2001) Coordinate control of sphingolipid biosynthesis and multidrug resistance in Saccharomyces cerevisiae. J Biol Chem 276(26):23674–23680. https://doi.org/10.1074/jbc.M101568200
Holford M, Daly M, King GF, Norton RS (2018) Venoms to the rescue. Science 361:842–844. https://doi.org/10.1126/science.aau7761
Holm T, Bruchmann J, Scheynius A, Langel U (2012) Cell-penetrating peptides as antifungals towards Malassezia sympodialis. Lett Appl Microbiol 54(1):39–44. https://doi.org/10.1111/j.1472-765X.2011.03168.x
Hossen S, Gan SH, Khalil I (2017) Melittin, a potential natural toxin of crude bee venom: probable future arsenal in the treatment of diabetes mellitus. J Chem 2017:1–7. https://doi.org/10.1155/2017/4035626
Hwang B, Hwang JS, Lee J, Lee DG (2010) Antifungal properties and mode of action of psacotheasin, a novel knottin-type peptide derived from Psacothea hilaris. Biochem Biophys Res Commun 400(3):352–357. https://doi.org/10.1016/j.bbrc.2010.08.063
Ji F, He D, Olaniran AO, Mokoena MP, Xu J, Shi J (2019) Occurrence, toxicity, production and detection of Fusarium mycotoxin: a review. Food Prod Process Nutr 1:6. https://doi.org/10.1186/s43014-019-0007-2
Jia F, Wang J, Peng J, Zhao P, Kong Z, Wang K, Yan W, Wang R (2018) The in vitro, in vivo antifungal activity and the action mode of Jelleine-I against Candida species. Amino Acids 50(2):229–239. https://doi.org/10.1007/s00726-017-2507-1
Karathia H, Vilaprinyo E, Sorribas A, Alves R (2011) Saccharomyces cerevisiae as a model organism: a comparative study. PLoS One 6(2):e16015. https://doi.org/10.1371/journal.pone.0016015
Kim DH, Lee DG, Kim KL, Lee Y (2001) Internalization of tenecin 3 by a fungal cellular process is essential for its fungicidal effect on Candida albicans. Eur J Biochem 268(16):4449–4458. https://doi.org/10.1046/j.1432-1327.2001.02364.x
Kim JH, Park J, Park KK, An HJ, Lee YW (2019) Evaluation of antifungal activities of bee venom components against Malassezia strains. J Mycol Infect 24(4):91–95. https://doi.org/10.17966/JMI.2019.24.4.91
Kredics L, Chen L, Kedves O, Büchner R, Hatvani L, Allaga H, Nagy VD, Khaled JM, Alharbi NS, Vágvölgyi C (2018) Molecular tools for monitoring Trichoderma in agricultural environments. Front Microbiol 9:1599. https://doi.org/10.3389/fmicb.2018.01599
Kumar N, Meena RC, Chakrabarti A (2011) Over-expression of YLR162W in Saccharomyces cerevisiae inhibits cell proliferation and renders cells susceptible to the hypoxic conditions induced by cobalt chloride. Indian J Microbiol 51(2):206–211. https://doi.org/10.1007/s12088-011-0132-3
Latgé JP, Chamilos G (2019) Aspergillus fumigatus and Aspergillosis in 2019. Clin Microbiol Rev 33(1):e00140–e00118. https://doi.org/10.1128/CMR.00140-18
Lee G, Bae H (2016) Anti-inflammatory applications of melittin, a major component of bee venom: detailed mechanism of action and adverse effects. Molecules 21(5). https://doi.org/10.3390/molecules21050616
Lee J, Lee DG (2009) Antifungal properties of a peptide derived from the signal peptide of the HIV-1 regulatory protein, Rev. FEBS Lett 583(9):1544–1547. https://doi.org/10.1016/j.febslet.2009.03.063
Lee J, Lee DG (2010) Influence of the hydrophobic amino acids in the N- and C-terminal regions of pleurocidin on antifungal activity. J Microbiol Biotechnol 20(8):1192–1195. https://doi.org/10.4014/jmb.1004.04041
Lee J, Lee DG (2014) Melittin triggers apoptosis in Candida albicans through the reactive oxygen species-mediated mitochondria/caspase-dependent pathway. FEMS Microbiol Lett 355(1):36–42. https://doi.org/10.1111/1574-6968.12450
Lee W, Lee DG (2015) Fungicidal mechanisms of the antimicrobial peptide Bac8c. Biochim Biophys Acta 1848(2):673–679. https://doi.org/10.1016/j.bbamem.2014.11.024
Lee MK, Lee DG, Shin SY, Lee SG, Kang JH, Hahm KS (1996) Antifungal activities of peptides with the sequence 10-17 of magainin 2 at the N-termini against Aspergillus fumigatus. J Microbiol 34(3):274–278
Lee DG, Park JH, Shin SY, Lee SG, Lee MK, Kim KL, Hahm KS (1997) Design of novel analogue peptides with potent fungicidal but low hemolytic activity based on the cecropin A-melittin hybrid structure. Biochem Mol Biol Int 43(3):489–498. https://doi.org/10.1080/15216549700204281
Lee DG, Shin SY, Maeng CY, Hahm KS (1998) Cecropin A-melittin hybrid peptide exerts its antifungal effects by damaging on the plasma membranes of Trichosporon beigelii. Biotechnol Lett 20:211–214. https://doi.org/10.1023/A:1005357314588
Lee DG, Kim DH, Park Y, Kim HK, Kim HN, Shin YK, Choi CH, Hahm KS (2001) Fungicidal effect of antimicrobial peptide, PMAP-23, isolated from porcine myeloid against Candida albicans. Biochem Biophys Res Commun 282(2):570–574. https://doi.org/10.1006/bbrc.2001.4602
Lee DG, Kim PI, Park Y, Jang SH, Park SC, Woo ER, Hahm KS (2002a) HP (2-20) derived from the amino terminal region of Helicobacter pylori ribosomal protein L1 exerts its antifungal effects by damaging the plasma membranes of Candida albicans. J Pept Sci 8(8):453–460. https://doi.org/10.1002/psc.405
Lee DG, Park Y, Kim HN, Kim HK, Kim PI, Choi BH, Hahm KS (2002b) Antifungal mechanism of an antimicrobial peptide, HP (2--20), derived from N-terminus of Helicobacter pylori ribosomal protein L1 against Candida albicans. Biochem Biophys Res Commun 291(4):1006–1013. https://doi.org/10.1006/bbrc.2002.6548
Lee DG, Kim HK, Kim SA, Park Y, Park SC, Jang SH (2003) Hahm KS (2003) Fungicidal effect of indolicidin and its interaction with phospholipid membranes. Biochem Biophys Res Commun 305(2):305–310. https://doi.org/10.1016/s0006-291x(03)00755-1
Lee J, Hwang JS, Hwang IS, Cho J, Lee E, Kim Y, Lee DG (2012) Coprisin-induced antifungal effects in Candida albicans correlate with apoptotic mechanisms. Free Radic Biol Med 52(11-12):2302–2311. https://doi.org/10.1016/j.freeradbiomed.2012.03.012
López-García B, Pérez-Payá E, Marcos JF (2002) Identification of novel hexapeptides bioactive against phytopathogenic fungi through screening of a synthetic peptide combinatorial library. Appl Environ Microbiol 68(5):2453–2460. https://doi.org/10.1128/aem.68.5.2453-2460.2002
López-García B, Gandía M, Muñoz A, Carmona L, Marcos JF (2010) A genomic approach highlights common and diverse effects and determinants of susceptibility on the yeast Saccharomyces cerevisiae exposed to distinct antimicrobial peptides. BMC Microbiol 10:289. https://doi.org/10.1186/1471-2180-10-289
Mao J, Liu S, Ai M, Wang Z, Wang D, Li X, Hu K, Gao X, Yang Y (2017) A novel melittin nano-liposome exerted excellent anti-hepatocellular carcinoma efficacy with better biological safety. J Hematol Oncol 10(1):71. https://doi.org/10.1186/s13045-017-0442-y
Martinez-Rossi NM, Bitencourt TA, Peres NTA, Lang EAS, Gomes EV, Quaresemin NR, Martins MP, Lopes L, Rossi A (2018) Dermatophyte resistance to antifungal drugs: mechanisms and prospectus. Front Microbiol 9:1108. https://doi.org/10.3389/fmicb.2018.01108
Matsuzaki K, Yoneyama S, Miyajima K (1997) Pore formation and translocation of melittin. Biophys J 73(2):831–838. https://doi.org/10.1016/S0006-3495(97)78115-3
Mazur P, Baginsky W (1996) In vitro activity of 1,3-beta-D-glucan synthase requires the GTP-binding protein Rho1. J Biol Chem 271(24):14604–14609. https://doi.org/10.1074/jbc.271.24.14604
Memariani H, Memariani M, Pourmand MR (2018) Venom-derived peptide Mastoparan-1 eradicates planktonic and biofilm-embedded methicillin-resistant Staphylococcus aureus isolates. Microb Pathog 119:72–80. https://doi.org/10.1016/j.micpath.2018.04.008
Memariani H, Memariani M, Ghasemian A (2019a) An overview on anti-biofilm properties of quercetin against bacterial pathogens. World J Microbiol Biotechnol 35(9):143. https://doi.org/10.1007/s11274-019-2719-5
Memariani H, Memariani M, Shahidi-Dadras M, Nasiri S, Akhavan MM, Moravvej H (2019b) Melittin: from honeybees to superbugs. Appl Microbiol Biotechnol 103(8):3265–3276. https://doi.org/10.1007/s00253-019-09698-y
Memariani H, Memariani M, Moravvej H, Shahidi-Dadras M (2020) Melittin: a venom-derived peptide with promising anti-viral properties. Eur J Clin Microbiol Infect Dis 39(1):5–17. https://doi.org/10.1007/s10096-019-03674-0
Moerman L, Bosteels S, Noppe W, Willems J, Clynen E, Schoofs L, Thevissen K, Tytgat J, Van Eldere J, Van Der Walt J, Verdonck F (2002) Antibacterial and antifungal properties of alpha-helical, cationic peptides in the venom of scorpions from southern Africa. Eur J Biochem 269(19):4799–4810. https://doi.org/10.1046/j.1432-1033.2002.03177.x
Moore J, Rajasekaran K, Cary JW, Chlan C (2019) Mode of action of the antimicrobial peptide D4E1 on Aspergillus flavus. Int J Pept Res Ther 25:1135–1145. https://doi.org/10.1007/s10989-018-9762-1
Moreno M, Giralt E (2015) Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: melittin, apamin and mastoparan. Toxins (Basel) 7(4):1126–1150. https://doi.org/10.3390/toxins7041126
Muñoz A, López-García B, Marcos JF (2006) Studies on the mode of action of the antifungal hexapeptide PAF26. Antimicrob Agents Chemother 50(11):3847–3855. https://doi.org/10.1128/AAC.00650-06
Neumann W, Habermann E, Amend G (1952) Zur papierelektrophoretischen fraktionierung tierischer gifte. Naturwissenschaften 39(12):286–287. https://doi.org/10.1007/BF00591257
Othon CM, Kwon OH, Lin MM, Zewail AH (2009) Solvation in protein (un)folding of melittin tetramer-monomer transition. Proc Natl Acad Sci U S A 106(31):12593–12598. https://doi.org/10.1073/pnas.0905967106
Park C, Lee DG (2009) Fungicidal effect of antimicrobial peptide arenicin-1. Biochim Biophys Acta 1788(9):1790–1796. https://doi.org/10.1016/j.bbamem.2009.06.008
Park C, Lee DG (2010) Melittin induces apoptotic features in Candida albicans. Biochem Biophys Res Commun 394(1):170–172. https://doi.org/10.1016/j.bbrc.2010.02.138
Park SC, Kim JY, Shin SO, Jeong CY, Kim MH, Shin SY, Cheong GW, Park Y, Hahm KS (2006) Investigation of toroidal pore and oligomerization by melittin using transmission electron microscopy. Biochem Biophys Res Commun 343(1):222–228. https://doi.org/10.1016/j.bbrc.2006.02.090
Park C, Cho J, Lee J, Lee DG (2011) Membranolytic antifungal activity of arenicin-1 requires the N-terminal tryptophan and the beta-turn arginine. Biotechnol Lett 33(1):185–189. https://doi.org/10.1007/s10529-010-0402-x
Park SC, Cheong MS, Kim EJ, Kim JH, Chi YH, Jang MK (2017) Antifungal effect of Arabidopsis SGT1 proteins via mitochondrial reactive oxygen species. J Agric Food Chem 65(38):8340–8347. https://doi.org/10.1021/acs.jafc.7b02808
Park SC, Kim JY, Kim EJ, Cheong GW, Lee Y, Choi W, Lee JR, Jang MK (2018a) Hydrophilic linear peptide with histidine and lysine residues as a key factor affecting antifungal activity. Int J Mol Sci 19(12):3781. https://doi.org/10.3390/ijms19123781
Park SC, Kim IR, Kim JY, Lee Y, Kim EJ, Jung JH, Jung YJ, Jang MK, Lee JR (2018b) Molecular mechanism of Arabidopsis thaliana profilins as antifungal proteins. Biochim Biophys Acta Gen Subj 1862(12):2545–2554. https://doi.org/10.1016/j.bbagen.2018.07.028
Pashaei F, Bevalian P, Akbari R, Pooshang Bagheri K (2019) Single dose eradication of extensively drug resistant Acinetobacter spp. in a mouse model of burn infection by melittin antimicrobial peptide. Microb Pathog 127:60–69. https://doi.org/10.1016/j.micpath.2018.11.055
Pennington MW, Czerwinski A, Norton RS (2018) Peptide therapeutics from venom: current status and potential. Bioorg Med Chem 26(10):2738–2758. https://doi.org/10.1016/j.bmc.2017.09.029
Perfect JR (2017) The antifungal pipeline: a reality check. Nat Rev Drug Discov 16(9):603–616. https://doi.org/10.1038/nrd.2017.46
Petrasch S, Knapp SJ, van Kan JAL, Blanco-Ulate B (2019) Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol Plant Pathol 20(6):877–892. https://doi.org/10.1111/mpp.12794
Pettit RK (2009) Mixed fermentation for natural product drug discovery. Appl Microbiol Biotechnol 83(1):19–25. https://doi.org/10.1007/s00253-009-1916-9
Piepers S, De Vliegher S, Demeyere K, Lambrecht BN, de Kruif A, Meyer E, Opsomer G (2009) Technical note: flow cytometric identification of bovine milk neutrophils and simultaneous quantification of their viability. J Dairy Sci 92(2):626–631. https://doi.org/10.3168/jds.2008-1393
Rady I, Siddiqui IA, Rady M, Mukhtar H (2017) Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett 402:16–31. https://doi.org/10.1016/j.canlet.2017.05.010
Raghuraman H, Chattopadhyay A (2007) Melittin: a membrane-active peptide with diverse functions. Biosci Rep 27(4–5):189–223. https://doi.org/10.1007/s10540-006-9030-z
Ramage G, Rajendran R, Sherry L, Williams C (2012) Fungal biofilm resistance. Int J Microbiol 2012:528521–528514. https://doi.org/10.1155/2012/528521
Roche CM, Loros JJ, McCluskey K, Glass NL (2014) Neurospora crassa: looking back and looking forward at a model microbe. Am J Bot 101(12):2022–2035. https://doi.org/10.3732/ajb.1400377
Schendel V, Rash LD, Jenner RA, Undheim EAB (2019) The diversity of venom: the importance of behavior and venom system morphology in understanding its ecology and evolution. Toxins (Basel) 11(11):666. https://doi.org/10.1126/10.3390/toxins11110666
Shin SH, Ye MK, Choi SY, Park KK (2017) The effects of melittin and apamin on airborne fungi-induced chemical mediator and extracellular matrix production from nasal polyp fibroblasts. Toxins (Basel) 2017:9(11). https://doi.org/10.3390/toxins9110348
Soman NR, Baldwin SL, Hu G, Marsh JN, Lanza GM, Heuser JE, Arbeit JM, Wickline SA, Schlesinger PH (2009) Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. J Clin Invest 119(9):2830–2842. https://doi.org/10.1172/JCI38842
Sommer A, Fries A, Cornelsen I, Speck N, Koch-Nolte F, Gimpl G, Andrä J, Bhakdi S, Reiss K (2012) Melittin modulates keratinocyte function through P2 receptor-dependent ADAM activation. J Biol Chem 287(28):23678–23689. https://doi.org/10.1074/jbc.M112.362756
Stephens C, Harrison SJ, Kazan K, Smith FW, Goulter KC, Maclean DJ, Manners JM (2005) Altered fungal sensitivity to a plant antimicrobial peptide through over-expression of yeast cDNAs. Curr Genet 47(3):194–201. https://doi.org/10.1007/s00294-005-0562-8
Stock SD, Hama H, Radding JA, Young DA, Takemoto JY (2000) Syringomycin E inhibition of Saccharomyces cerevisiae: requirement for biosynthesis of sphingolipids with very-long-chain fatty acids and mannose- and phosphoinositol-containing head groups. Antimicrob Agents Chemother 44(5):1174–1180. https://doi.org/10.1128/aac.44.5.1174-1180.2000
Su H, Han L, Huang X (2018) Potential targets for the development of new antifungal drugs. J Antibiot (Tokyo) 71(12):978–991. https://doi.org/10.1038/s41429-018-0100-9
Sung WS, Lee DG (2008) Pleurocidin-derived antifungal peptides with selective membrane-disruption effect. Biochem Biophys Res Commun 369(3):858–861. https://doi.org/10.1016/j.bbrc.2008.02.109
Thayikkannu AB, Kindo AJ, Veeraraghavan M (2015) Malassezia-can it be ignored? Indian J Dermatol 60(4):332–339. https://doi.org/10.4103/0019-5154.160475
Thevissen K, Cammue BP, Lemaire K, Winderickx J, Dickson RC, Lester RL, Ferket KK, Van Even F, Parret AH, Broekaert WF (2000) A gene encoding a sphingolipid biosynthesis enzyme determines the sensitivity of Saccharomyces cerevisiae to an antifungal plant defensin from dahlia (Dahlia merckii). Proc Natl Acad Sci U S A 97(17):9531–9536. https://doi.org/10.1073/pnas.160077797
Tomasinsig L, Scocchi M, Mettulio R, Zanetti M (2004) Genome-wide transcriptional profiling of the Escherichia coli response to a proline-rich antimicrobial peptide. Antimicrob Agents Chemother 48(9):3260–3267. https://doi.org/10.1128/AAC.48.9.3260-3267.2004
Tosteson MT, Holmes SJ, Razin M, Tosteson DC (1985) Melittin lysis of red cells. J Membr Biol 87(1):35–44. https://doi.org/10.1007/BF01870697
Author information
Authors and Affiliations
Contributions
HM contributed to the conception of the study and data collection. MM and HM jointly analyzed the data and wrote the manuscript. Both authors have critically reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Memariani, H., Memariani, M. Anti-fungal properties and mechanisms of melittin. Appl Microbiol Biotechnol 104, 6513–6526 (2020). https://doi.org/10.1007/s00253-020-10701-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10701-0