Abstract
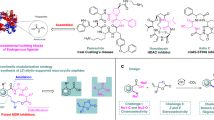
The synthesis of rhamnosylated compounds has gained great importance since these compounds have potential therapeutic applications. The enzymatic approaches for glycosylation of bioactive molecules have been well developed; however, the enzymatic rhamnosylation has been largely hindered by lacking of the glycosyl donor for rhamnosyltransferases. Here, we employed an α-l-rhamnosidase from Alternaria sp. L1 (RhaL1) to perform one-step rhamnosylation of anticancer drugs, including 2′-deoxy-5-fluorouridine (FUDR), cytosine arabinoside (Ara C), and hydroxyurea (Hydrea). The key synthesis conditions including substrate concentrations and reaction time were carefully optimized, and the maximum yields of each rhamnosylated drugs were 57.7 mmol for rhamnosylated Ara C, 68.6 mmol for rhamnosylated Hydrea, and 42.2 mmol for rhamnosylated FUDR. It is worth pointing out that these rhamnosylated drugs exhibit little cytotoxic effects on cancer cells, but could efficiently restore cytotoxic activity when incubated with exogenous α-l-rhamnosidase, suggesting their potential applications in the enzyme-activated prodrug system. To evaluate the cancer-targeting ability of rhamnose moiety, the rhamnose-conjugated fluorescence dye rhodamine B (Rha-RhB) was constructed. The fluorescence probe Rha-RhB displayed much higher cell affinity and cellular internalization rate of oral cancer cell KB and breast cancer cell MDA-MB-231 than that of the normal epithelial cells MCF 10A, suggesting that the rhamnose moiety could mediate the specific internalization of rhamnosylated compounds into cancer cells, which greatly facilitated their applications for cancer-targeting drug delivery.





Similar content being viewed by others
References
Abraham R, Aman N, von Borstel R, Darsley M, Kamireddy B, Kenten J, Morris G, Titmas R (1994) Conjugates of COL-1 monoclonal antibody and β-D-galactosidase can specifically kill tumor cells by generation of 5-fluorouridine from the prodrug β-D-galactosyl-5-fluorouridine. Cell Biophys 24(1):127–133. https://doi.org/10.1007/bf02789223
Andreotti G, Trincone A, Giordano A (2007) Convenient synthesis of β-galactosyl nucleosides using the marine β-galactosidase from Aplysia fasciata. J Mol Catal, B Enzym 47(1):28–32
Binder WH, Kählig H, Schmid W (1995) Galactosylation by use of β-galactosidase: enzymatic syntheses of disaccharide nucleosides. Tetrahedron: Asymmetry 6(7):1703–1710. https://doi.org/10.1016/0957-4166(95)00216-C
Burger MM, Glaser L, Burton RM (1963) The enzymatic synthesis of a rhamnose-containing glycolipid by extracts of Pseudomonas aeruginosa. J Biol Chem 238(8):2595–2602
Cham B (2013) Drug therapy: solamargine and other solasodine rhamnosyl glycosides as anticancer agents. Modern Chemotherapy 2:33–49. https://doi.org/10.4236/mc.2013.22005
Crout DH, Vic G (1998) Glycosidases and glycosyl transferases in glycoside and oligosaccharide synthesis. Curr Opin Chem Biol 2(1):98–111
Danby PM, Withers SG (2016) Advances in enzymatic glycoside synthesis. ACS Chem Biol 11(7):1784–1794. https://doi.org/10.1021/acschembio.6b00340
De Winter K, Šimčíková D, Schalck B, Weignerová L, Pelantova H, Soetaert W, Desmet T, Křen V (2013) Chemoenzymatic synthesis of α-L-rhamnosides using recombinant α-L-rhamnosidase from Aspergillus terreus. Bioresour Technol 147(Supplement C):640–644. https://doi.org/10.1016/j.biortech.2013.08.083
Filice M, Palomo JM (2012) Monosaccharide derivatives as central scaffolds in the synthesis of glycosylated drugs. RSC Advances 2(5):1729–1742. https://doi.org/10.1039/c2ra00515h
Garnier P, Wang XT, Robinson MA, van Kasteren S, Perkins AC, Frier M, Fairbanks AJ, Davis BG (2010) Lectin-directed enzyme activated prodrug therapy (LEAPT): synthesis and evaluation of rhamnose-capped prodrugs. J Drug Target 18(10):794–802. https://doi.org/10.3109/1061186X.2010.529909
Giang I, Boland EL, Poon GMK (2014) Prodrug applications for targeted cancer therapy. AAPS J 16(5):899–913. https://doi.org/10.1208/s12248-014-9638-z
Iglesias LE, Lewkowicz ES, Medici R, Bianchi P, Iribarren AM (2015) Biocatalytic approaches applied to the synthesis of nucleoside prodrugs. Biotechnol Adv 33(5):412–434. https://doi.org/10.1016/j.biotechadv.2015.03.009
Irache JM, Salman HH, Gamazo C, Espuelas S (2008) Mannose-targeted systems for the delivery of therapeutics. Expert Opin Drug Deliv 5(6):703–724. https://doi.org/10.1517/17425247.5.6.703
Julio P, Andrew PM (2007) Industrial enzymes structure, function and applications. Springer, Netherlands, pp 142–143. https://doi.org/10.1007/1-4020-5377-0
Li N, Smith TJ, Zong MH (2010) Biocatalytic transformation of nucleoside derivatives. Biotechnol Adv 28(3):348–366. https://doi.org/10.1016/j.biotechadv.2010.01.006
Li S, Wang H, Ma J, Gu G, Chen Z, Guo Z (2016) One-pot four-enzyme synthesis of thymidinediphosphate-L-rhamnose. Chem Commun 52(97):13995–13998. https://doi.org/10.1039/c6cc08366h
Lipscombe RJ, Carter SJ, Ruane M (2005) Rhamnose binding protein. US6930171B2. Solbec Pharmaceuticals Limited, Nedlands (AU)
Lu L, Liu Q, Jin L, Yin Z, Xu L, Xiao M (2015) Enzymatic synthesis of rhamnose containing chemicals by reverse hydrolysis. PLoS One 10(10):e0140531. https://doi.org/10.1371/journal.pone.0140531
Ma Y, Chen H, Su S, Wang T, Zhang C, Fida G, Cui S, Zhao J, Gu Y (2015) Galactose as broad ligand for multiple tumor imaging and therapy. J Cancer 6(7):658–670. https://doi.org/10.7150/jca.11647
Malanga M, Darcsi A, Balint M, Benkovics G, Sohajda T, Beni S (2016) New synthetic strategies for xanthene-dye-appended cyclodextrins. Beilstein J Org Chem 12:537–548. https://doi.org/10.3762/bjoc.12.53
Martearena MR, Blanco S, Ellenrieder G (2003) Synthesis of alkyl-alpha-L-rhamnosides by water soluble alcohols enzymatic glycosylation. Bioresour Technol 90(3):297–303
Martearena MR, Daz M, Ellenrieder G (2008) Synthesis of rutinosides and rutinose by reverse hydrolysis catalyzed by fungal α-L-rhamnosidases. Biocatal Biotransform 26(3):177–185. https://doi.org/10.1080/10242420701568617
Parajuli P, Pandey RP, Gurung RB, Shin JY, Jung HJ, Kim DH, Sohng JK (2016) Enzymatic synthesis of lactosylated and sialylated derivatives of epothilone A. Glycoconj J 33(2):137–146
Rather MY, Mishra S (2013) β-Glycosidases: an alternative enzyme based method for synthesis of alkyl-glycosides. Sustain Chem Processes 1(1):7. https://doi.org/10.1186/2043-7129-1-7
Robinson MA, Charlton ST, Garnier P, Wang X, Davis SS, Perkins AC, Frier M, Duncan R, Savage TJ, Wyatt DA, Watson SA, Davis BG (2004) LEAPT: Lectin-directed enzyme-activated prodrug therapy. Proc Natl Acad Sci U S A 101(40):14527–14532. https://doi.org/10.1073/pnas.0303574101
Sarkar S, Salyer AC, Wall KA, Sucheck SJ (2013) Synthesis and immunological evaluation of a MUC1 glycopeptide incorporated into l-rhamnose displaying liposomes. Bioconjug Chem 24(3):363–375. https://doi.org/10.1021/bc300422a
Šimčíková D, Kotik M, Weignerová L, Halada P, Pelantová H, Adamcová K, Křen V (2015) α-L-Rhamnosyl-β-D-glucosidase (rutinosidase) from Aspergillus niger: characterization and synthetic potential of a novel diglycosidase. Adv Synth Catal 357(1):107–117. https://doi.org/10.1002/adsc.201400566
Singh S, Aggarwal A, Bhupathiraju NVSDK, Arianna G, Tiwari K, Drain CM (2015) Glycosylated porphyrins, phthalocyanines, and other porphyrinoids for diagnostics and therapeutics. Chem Rev 115(18):10261–10306. https://doi.org/10.1021/acs.chemrev.5b00244
Suzuki Y, Uchida K (1994) Formation of beta-galactosyl compounds of arabinosylcytosine in growing culture of Sporobolomyces singularis. Biosci Biotechnol Biochem 58(4):639–643. https://doi.org/10.1271/bbb.58.639
Uchida K, Suzuki Y (2003) Formation of 3′-O-O-galactosyl compounds of 5-bromouridine by Sporobolomyces singularis. Biosci Biotechnol Biochem 67(3):643–645. https://doi.org/10.1271/bbb.67.643
van Rantwijk F, Woudenberg-van Oosterom M, Sheldon RA (1999) Glycosidase-catalysed synthesis of alkyl glycosides. J Mol Catal B: Enzym 6(6):511–532. https://doi.org/10.1016/S1381-1177(99)00042-9
Vankayala SL, Hargis JC, Woodcock HL (2012) Unlocking the binding and reaction mechanism of hydroxyurea substrates as biological nitric oxide donors. J Chem Inf Model 52(5):1288–1297. https://doi.org/10.1021/ci300035c
Wang Y, Gao J, Gu G, Li G, Cui C, Sun B, Lou H (2011) In situ RBL receptor visualization and its mediated anticancer activity for solasodine rhamnosides. ChemBioChem 12(16):2418–2420. https://doi.org/10.1002/cbic.201100551
Xu L, Liu X, Yin Z, Liu Q, Lu L, Xiao M (2016) Site-directed mutagenesis of alpha-L-rhamnosidase from Alternaria sp. L1 to enhance synthesis yield of reverse hydrolysis based on rational design. Appl Microbiol Biotechnol 100(24):10385–10394. https://doi.org/10.1007/s00253-016-7676-4
Yan LQ, Li N, Zong MH (2013) First and facile enzymatic synthesis of β-fucosyl-containing disaccharide nucleosides through β-galactosidase-catalyzed regioselective glycosylation. J Biotechnol 164(2):371–375. https://doi.org/10.1016/j.jbiotec.2013.01.024
Yan LQ, Li N, Zong MH (2014) First enzymatic galactosylation of acyclic nucleoside drugs by β-galactosidase: Synthesis of water-soluble β-D-galactosidic prodrugs. Biotechnol Bioprocess Eng 19(4):586–591. https://doi.org/10.1007/s12257-013-0823-1
Funding
This work was supported by the National Key Research and Development Program of China (2018YFA0902000), National Natural Science Foundation of China (31670062 and 31301155), the Science and Technology Development Project of Shandong Province (2016GGH4502 and 2017GSF21115), and the Fundamental Research Funds of Shandong University (2016JC028).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1894 kb)
Rights and permissions
About this article
Cite this article
Xu, L., Liu, X., Li, Y. et al. Enzymatic rhamnosylation of anticancer drugs by an α-l-rhamnosidase from Alternaria sp. L1 for cancer-targeting and enzyme-activated prodrug therapy. Appl Microbiol Biotechnol 103, 7997–8008 (2019). https://doi.org/10.1007/s00253-019-10011-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10011-0