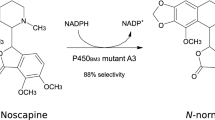
Abstract
Tetrahydroprotoberberines (THPBs), a class of naturally occurring isoquinoline alkaloids, contain substituent methoxyl or hydroxyl groups which play a significant role in the pharmacological properties of these molecules. In this study, we report a biocatalytic strategy for selective O-demethylation of THPBs. CYP105D1, a cytochrome P450 from Streptomyces griseus ATCC 13273, exhibited markedly regioselective demethylation of nonhydroxyl-THPBs and monohydroxyl-THPBs on the D-ring. A possible binding mode of THPBs with CYP105D1 was investigated by docking analysis, and the results revealed that the D-rings of THPBs were with the minimum distance to the heme iron. Tetrahydropalmatine was used as a model substrate and enantioselective demethylation was demonstrated. (S)-Tetrahydropalmatine was only demethylated at C-10, while (R)-tetrahydropalmatine was first demethylated at C-10 and then subsequently demethylated at C-9. The kcat/Km value for demethylation of (R)-tetrahydropalmatine by CYP105D1 was 3.7 times greater than that for demethylation of (S)-tetrahydropalmatine. Furthermore, selective demethylation of (S)-tetrahydropalmatine by the CYP105D1-based whole-cell system was demonstrated for the highly efficient production of (S)-corydalmine which has distinct pharmacological applications, such as providing relief from bone cancer pain and reducing morphine tolerance. Moreover, a homologous redox partner was identified to enhance the catalytic efficiency of the CYP105D1-based whole-cell system. This is the first enzymatic characterization of a cytochrome P450 that has regio- and enantioselective demethylation activity of THPBs for application purpose. The cytochrome P450 system could be a promising strategy for selective demethylation in the pharmaceutical industry.









Similar content being viewed by others
References
Becher R, Wirsel SG (2012) Fungal cytochrome P450 sterol 14alpha-demethylase (CYP51) and azole resistance in plant and human pathogens. Appl Microbiol Biotechnol 95(4):825–840. https://doi.org/10.1007/s00253-012-4195-9
Belferdi F, Merabet N, Belkhiri L, Douara B (2016) Regioselective demethylation of quinoline derivatives. A DFT rationalization. J Mol Struct 1118:10–17. https://doi.org/10.1016/j.molstruc.2016.03.034
Bernhardt R (2006) Cytochromes P450 as versatile biocatalysts. J Biotechnol 124(1):128–145. https://doi.org/10.1016/j.jbiotec.2006.01.026
Chu H, Jin G, Friedman E, Zhen X (2008) Recent development in studies of tetrahydroprotoberberines: mechanism in antinociception and drug addiction. Cell Mol Neurobiol 28(4):491–499. https://doi.org/10.1007/s10571-007-9179-4
Chun YJ, Shimada T, Sanchez-Ponce R, Martin MV, Lei L, Zhao B, Kelly SL, Waterman MR, Lamb DC, Guengerich FP (2007) Electron transport pathway for a Streptomyces cytochrome P450: cytochrome P450 105D5-catalyzed fatty acid hydroxylation in Streptomyces coelicolor A3(2). J Biol Chem 282(24):17486–17500. https://doi.org/10.1074/jbc.M700863200
Coleman T, Chao RR, Bruning JB, De Voss JJ, Bell SG (2015) CYP199A4 catalyses the efficient demethylation and demethenylation of para-substituted benzoic acid derivatives. RSC Adv 5(64):52007–52018. https://doi.org/10.1039/c5ra08730a
Constable DJC, Dunn PJ, Hayler JD, Humphrey GR, Leazer JJL, Linderman RJ, Lorenz K, Manley J, Pearlman BA, Wells A, Zaks A, Zhang TY (2007) Key green chemistry research areas—a perspective from pharmaceutical manufacturers. Green Chem 9(5):411–420. https://doi.org/10.1039/b703488c
Dai WL, Xiong F, Yan B, Cao ZY, Liu WT, Liu JH, Yu BY (2016) Blockade of neuronal dopamine D2 receptor attenuates morphine tolerance in mice spinal cord. Sci Rep 6(1). https://doi.org/10.1038/srep38746
Dai WL, Yan B, Jiang N, Wu JJ, Liu XF, Liu JH, Yu BY (2017) Simultaneous inhibition of NMDA and mGlu1/5 receptors by levo-corydalmine in rat spinal cord attenuates bone cancer pain. Int J Cancer 141(4):805–815. https://doi.org/10.1002/ijc.30780
Dubey KK, Jawed A, Haque S (2013) Enhanced bioconversion of colchicine to regiospecific 3-demethylated colchicine (3-DMC) by whole cell immobilization of recombinant E. coli harboring P450 BM-3 gene. Process Biochem 48(8):1151–1158. https://doi.org/10.1016/j.procbio.2013.06.001
Fasan R (2017) Enzymatic catalysis: new functional twists for P450s. Nat Chem 9(7):609–611. https://doi.org/10.1038/nchem.2810
Girhard M, Klaus T, Khatri Y, Bernhardt R, Urlacher VB (2010) Characterization of the versatile monooxygenase CYP109B1 from Bacillus subtilis. Appl Microbiol Biotechnol 87(2):595–607. https://doi.org/10.1007/s00253-010-2472-z
Guo D, Li J, Lin H, Zhou Y, Chen Y, Zhao F, Sun H, Zhang D, Li H, Shoichet BK, Shan L, Zhang W, Xie X, Jiang H, Liu H (2016) Design, synthesis, and biological evaluation of novel tetrahydroprotoberberine derivatives (THPBs) as selective alpha1A-adrenoceptor antagonists. J Med Chem 59(20):9489–9502. https://doi.org/10.1021/acs.jmedchem.6b01217
Hlavica P (2009) Assembly of non-natural electron transfer conduits in the cytochrome P450 system: a critical assessment and update of artificial redox constructs amenable to exploitation in biotechnological areas. Biotechnol Adv 27(2):103–121. https://doi.org/10.1016/j.biotechadv.2008.10.001
Hussain HA, Ward JM (2003) Ferredoxin reductase enhances heterologously expressed cytochrome CYP105D1 in Escherichia coli and Streptomyces lividans. Enzyme Microb Technol 32(7):790–800. https://doi.org/10.1016/s0141-0229(03)00047-4
Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR (2011) Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev 63(3):772–810. https://doi.org/10.1124/pr.110.004135
Jin GZ, Zhu ZT, Fu Y (2002) (-)-Stepholidine: a potential novel antipsychotic drug with dual D1 receptor agonist and D2 receptor antagonist actions. Trends Pharmacol Sci 23(1):4–7
Jung E, Park BG, Ahsan MM, Kim J, Yun H, Choi KY, Kim BG (2016) Production of omega-hydroxy palmitic acid using CYP153A35 and comparison of cytochrome P450 electron transfer system in vivo. Appl Microbiol Biotechnol 100(24):10375–10384. https://doi.org/10.1007/s00253-016-7675-5
Khatri Y, Hannemann F, Ewen KM, Pistorius D, Perlova O, Kagawa N, Brachmann AO, Muller R, Bernhardt R (2010) The CYPome of Sorangium cellulosum So ce56 and identification of CYP109D1 as a new fatty acid hydroxylase. Chem Biol 17(12):1295–1305. https://doi.org/10.1016/j.chembiol.2010.10.010
Kozono I, Mihara K, Minagawa K, Hibi M, Ogawa J (2017) Engineering of the cytochrome P450 monooxygenase system for benzyl maltol hydroxylation. Appl Microbiol Biotechnol 101(17):6651–6658. https://doi.org/10.1007/s00253-017-8414-2
Lewis JC, Bastian S, Bennett CS, Fu Y, Mitsuda Y, Chen MM, Greenberg WA, Wong CH, Arnold FH (2009) Chemoenzymatic elaboration of monosaccharides using engineered cytochrome P450BM3 demethylases. PNAS 106(39):16550–16555. https://doi.org/10.1073/pnas.0908954106
Li L, Ye M, Bi K, Guo D (2006) Liquid chromatography-tandem mass spectrometry for the identification of L-tetrahydropalmatine metabolites in Penicillium janthinellum and rats. Biomed Chromatogr 20(1):95–100. https://doi.org/10.1002/bmc.534
Liu J, Yu B, Fei H, Dai W (2016) CN.Patent 105878239A. 2016-08-24
Ma B, Yue K, Chen L, Tian X, Ru Q, Gan Y, Wang D, Jin G, Li C (2014) L-stepholidine, a natural dopamine receptor D1 agonist and D2 antagonist, inhibits heroin-induced reinstatement. Neurosci Lett 559:67–71. https://doi.org/10.1016/j.neulet.2013.10.066
Mazzaferro LS, Huttel W, Fries A, Muller M (2015) Cytochrome P450-catalyzed regio- and stereoselective phenol coupling of fungal natural products. J Am Chem Soc 137(38):12289–12295. https://doi.org/10.1021/jacs.5b06776
Mo J, Guo Y, Yang YS, Shen JS, Jin GZ, Zhen X (2007) Recent developments in studies of l-stepholidine and its analogs: chemistry, pharmacology and clinical implications. Curr Med Chem 14(28):2996–3002. https://doi.org/10.2174/092986707782794050
Omura T, Sato R (1964) The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem 239:2370–2378
Pandey BP, Roh C, Choi KY, Lee N, Kim EJ, Ko S, Kim T, Yun H, Kim BG (2010) Regioselective hydroxylation of daidzein using P450 (CYP105D7) from Streptomyces avermitilis MA4680. Biotechnol Bioeng 105(4):697–704. https://doi.org/10.1002/bit.22582
Pandey BP, Lee N, Choi KY, Jung E, Jeong DH, Kim BG (2011) Screening of bacterial cytochrome P450s responsible for regiospecific hydroxylation of (iso)flavonoids. Enzym Microb Technol 48(4–5):386–392. https://doi.org/10.1016/j.enzmictec.2011.01.001
Pandey BP, Lee N, Choi KY, Kim JN, Kim EJ, Kim BG (2014) Identification of the specific electron transfer proteins, ferredoxin, and ferredoxin reductase, for CYP105D7 in Streptomyces avermitilis MA4680. Appl Microbiol Biotechnol 98(11):5009–5017. https://doi.org/10.1007/s00253-014-5525-x
Passiniemi M, Myllymaki MJ, Vuokko J, Koskinen AMP (2011) Demethylation of aromatic methyl ethers using ionic liquids under microwave irradiation. Lett Org Chem 8(1):48–52. https://doi.org/10.2174/157017811794557741
Peterson JA, Lorence MC, Amarneh B (1990) Putidaredoxin reductase and putidaredoxin. Cloning, sequence determination, and heterologous expression of the proteins. J Biol Chem 265(11):6066–6073
Putkaradze N, Litzenburger M, Abdulmughni A, Milhim M, Brill E, Hannemann F, Bernhardt R (2017) CYP109E1 is a novel versatile statin and terpene oxidase from Bacillus megaterium. Appl Microbiol Biotechnol 101(23–24):8379–8393. https://doi.org/10.1007/s00253-017-8552-6
Ran N, Zhao L, Chen Z, Tao J (2008) Recent applications of biocatalysis in developing green chemistry for chemical synthesis at the industrial scale. Green Chem 10(4):361–372. https://doi.org/10.1039/b716045c
Richter N, Zepeck F, Kroutil W (2015) Cobalamin-dependent enzymatic O-, N-, and S-demethylation. Trends Biotechnol 33(7):371–373. https://doi.org/10.1016/j.tibtech.2015.03.011
Sun H, Zhu L, Yang H, Qian W, Guo L, Zhou S, Gao B, Li Z, Zhou Y, Jiang H, Chen K, Zhen X, Liu H (2013a) Asymmetric total synthesis and identification of tetrahydroprotoberberine derivatives as new antipsychotic agents possessing a dopamine D(1), D(2) and serotonin 5-HT(1A) multi-action profile. Bioorg Med Chem 21(4):856–868. https://doi.org/10.1016/j.bmc.2012.12.016
Sun SY, Wang YQ, Li LP, Wang L, Zeng S, Zhou H, Jiang HD (2013b) Stereoselective interaction between tetrahydropalmatine enantiomers and CYP enzymes in human liver microsomes. Chirality 25(1):43–47. https://doi.org/10.1002/chir.22110
Tang X, Di X, Zhong Z, Xie Q, Chen Y, Wang F, Ling Z, Xu P, Zhao K, Wang Z, Liu L, Liu X (2016) In vitro metabolism of l-corydalmine, a potent analgesic drug, in human, cynomolgus monkey, beagle dog, rat and mouse liver microsomes. J Pharm Biomed Anal 128:98–105. https://doi.org/10.1016/j.jpba.2016.05.024
Taylor M, Lamb DC, Cannell R, Dawson M, Kelly SL (1999) Cytochrome P450105D1 (CYP105D1) from Streptomyces griseus: heterologous expression, activity, and activation effects of multiple xenobiotics. Biochem Biophys Res Commun 263(3):838–842. https://doi.org/10.1006/bbrc.1999.1427
Taylor M, Lamb DC, Cannell RJ, Dawson MJ, Kelly SL (2000) Cofactor recycling with immobilized heterologous cytochrome P450 105D1 (CYP105D1). Biochem Biophys Res Commun 279(2):708–711. https://doi.org/10.1006/bbrc.2000.4002
Trower MK, Sariaslani FS, O'Keefe DP (1989) Purification and characterization of a soybean flour-induced cytochrome P-450 from Streptomyces griseus. J Bacteriol 171(4):7
Trower MK, Lenstra R, Omer C, Buchholz SE, Sariaslani FS (1992) Cloning, nucleotide sequence determination and expression of the genes encoding cytochrome P-450soy (soyC) and ferredoxin soy (soyB) from Streptomyces griseus. Mol Microbiol 6(15):2125–2134
Ueno M, Yamashita M, Hashimoto M, Hino M, Fujie A (2005) Oxidative activities of heterologously expressed CYP107B1 and CYP105D1 in whole-cell biotransformation using Streptomyces lividans TK24. J Biosci Bioeng 100(5):567–572. https://doi.org/10.1263/jbb.100.567
Wang W, Liu J, Zhao X, Peng Y, Wang N, Lee DYW, Dai R (2017) Simultaneous determination of l-tetrahydropalmatine and its active metabolites in rat plasma by a sensitive ultra-high-performance liquid chromatography with tandem mass spectrometry method and its application in a pharmacokinetic study. Biomed Chromatogr 31(6):e3903. https://doi.org/10.1002/bmc.3903
Wei Y, Ang EL, Zhao H (2018) Recent developments in the application of P450 based biocatalysts. Curr Opin Chem Biol 43:1–7. https://doi.org/10.1016/j.cbpa.2017.08.006
Yu B, Zhang W, Liu J, Wang H, Chen Y (2002) CN. Patent. 1164759C. 2004-09-01
Zhang Y, Shi K, Wen J, Fan G, Chai Y, Hong Z (2012) Chiral HPLC determination and stereoselective pharmacokinetics of tetrahydroberberine enantiomers in rats. Chirality 24(3):239–244. https://doi.org/10.1002/chir.21988
Zhang MX, Hu XH, Xu YH, Loh TP (2015) Selective dealkylation of alkyl aryl ethers. Asian J Org Chem 4(10):1047–1049. https://doi.org/10.1002/ajoc.201500196
Funding
This work was supported by the Major Scientific and Technological Specialized Project for “New Drugs Development” (No. 2012ZX09J12110-06B) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 1211 kb)
Rights and permissions
About this article
Cite this article
Shen, C., Shan, T., Zhao, W. et al. Regio- and enantioselective O-demethylation of tetrahydroprotoberberines by cytochrome P450 enzyme system from Streptomyces griseus ATCC 13273. Appl Microbiol Biotechnol 103, 761–776 (2019). https://doi.org/10.1007/s00253-018-9416-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9416-4