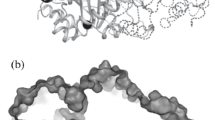
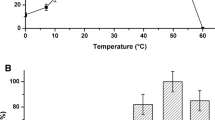
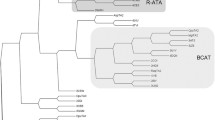
Abstract
Substrate and reaction promiscuity is a remarkable property of some enzymes and facilitates the adaptation to new metabolic demands in the evolutionary process. Substrate promiscuity is also a basis for protein engineering for biocatalysis. However, molecular principles of enzyme promiscuity are not well understood. Even for the widely studied PLP-dependent transaminases of class III, the reliable prediction of the biocatalytically important amine transaminase activity is still difficult if the desired activity is unrelated to the natural activity. Here, we show that 7,8-diaminopelargonic acid transaminase (synthase), previously considered to be highly specific, is able to convert (S)-(-)-1-phenylethylamine and a number of aldehydes and diketones. We were able to characterize the (S)-amine transaminase activity of 7,8-diaminopelargonic acid transaminase from Psychrobacter cryohalolentis (Pcryo361) and analyzed the three-dimensional structure of the enzyme. New substrate specificity for α-diketones was observed, though only a weak activity towards pyruvate was found. We examined the organization of the active site and binding modes of S-adenosyl-L-methionine and (S)-(-)-1-phenylethylamine using X-ray analysis and molecular docking. We suggest that the Pcryo361 affinity towards (S)-(-)-1-phenylethylamine arises from the recognition of the hydrophobic parts of the specific substrates, S-adenosyl-L-methionine and 7-keto-8-aminopelargonic acid, and from the flexibility of the active site. Our results support the observation that the conversion of amines is a promiscuous activity of many transaminases of class III and is independent from their natural function. The analysis of amine transaminase activity from among various transaminases will help to make the sequence-function prediction for biocatalysis more reliable.




Similar content being viewed by others
References
Bakermans C, Ayala-del-Río HL, Ponder MA, Vishnivetskaya T, Gilichinsky D, Thomashow MF, Tiedje JM (2006) Psychrobacter cryohalolentis sp. nov. and Psychrobacter arcticus sp. nov., isolated from Siberian permafrost. Int J Syst Evol Microbiol 56:1285–1291. https://doi.org/10.1099/ijs.0.64043-0
Battye TGG, Kontogiannis L, Johnson O, Powell HR, Leslie AGW (2011) iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr D Biol Crystallogr 67:271–281. https://doi.org/10.1107/S0907444910048675
Bea H-S, Park H-J, Lee S-H, Yun H (2011) Kinetic resolution of aromatic β-amino acids by ω-transaminase. Chem Commun 47:5894–5896. https://doi.org/10.1039/c1cc11528f
Bezsudnova EY, Stekhanova TN, Suplatov DA, Mardanov AV, Ravin NV, Popov VO (2016) Experimental and computational studies on the unusual substrate specificity of branched-chain amino acid aminotransferase from Thermoproteus uzoniensis. Arch Biochem Biophys 607:27–36. https://doi.org/10.1016/j.abb.2016.08.009
Boratyn GM, Camacho C, Cooper PS, Coulouris G, Fong A, Ma N, Madden TL, Matten WT, McGinnis SD, Merezhuk Y, Raytselis Y, Sayers EW, Tao T, Ye J, Zaretskaya I (2013) BLAST: a more efficient report with usability improvements. Nucleic Acids Res 41:W29–W33. https://doi.org/10.1093/nar/gkt282
Boyko KM, Gorbacheva MA, Rakitina TV, Korgenevskiy DA, Vanyushkina AA, Kamashev DE, Lipkin AV, Popov VO (2015) Expression, purification, crystallization and preliminary X-ray crystallographic analysis of histone-like HU-protein from Spiroplasma melliferum KC3. Acta Crystallogr F 71:24–27. https://doi.org/10.1107/S2053230X14025333
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Chem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Breen RS, Campopiano DJ, Webster S, Brunton M, Watt R, Baxter RL (2003) The mechanism of 7,8-diaminopelargonate synthase: the role of S-adenosylmethionine as the amino donor. Org Biomol Chem 1:3498–3499. https://doi.org/10.1039/b310443p
Cobessi D, Dumas R, Pautre V, Meinguet C, Ferrer JL, Alban C (2012) Biochemical and structural characterization of the Arabidopsis bifunctional enzyme dethiobiotin synthetase–diaminopelargonic acid aminotransferase: evidence for substrate channeling in biotin synthesis. Plant Cell 24:1608–1625. https://doi.org/10.1105/tpc.112.097675
Dey S, Lane JM, Lee RE, Rubin EJ, Sacchettini JC (2010) Structural characterization of the Mycobacterium tuberculosis biotin biosynthesis enzymes 7,8-diaminopelargonic acid synthase and dethiobiotinsynthetase. Biochemistry 49:6746–6760. https://doi.org/10.1021/bi902097j
Diederichs K, Karplus PA (1997) Improved R-factors for diffraction data analysis in macromolecular crystallography. Nat Struct biol 4:269-275. Erratum in: Nat Struct biol 1997 4:592. doi: https://doi.org/10.1038/nsb0497-269
Dolinsky TJ, Nielsen JE, McCammon JA, Baker NA (2004) PDB2PQR: an automated pipeline for the setup, execution, and analysis of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res 32:W665–W667. https://doi.org/10.1093/nar/gkh381
Eliot AC, Kirsch JF (2004) Pyridoxal phosphate enzymes: mechanistic, structural, and evolutionary considerations. Annu Rev Biochem 73:383–415. https://doi.org/10.1146/annurev.biochem.73.011303.074021
Eliot AC, Sandmark J, Schneider G, Kirsch JF (2002) The dual-specific active site of 7,8-diaminopelargonic acid synthase and the effect of the R391A mutation. Biochemistry 41:12582–12589. https://doi.org/10.1021/bi026339a
Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66:486–501. https://doi.org/10.1107/S0907444910007493
Evans P (2006) Scaling and assessment of data quality. Acta Crystallogr D Biol Crystallogr 62:72–82. https://doi.org/10.1107/S0907444905036693
Fiser A, Sali A (2003) Modeller: generation and refinement of homology-based protein structure models. Methods Enzymol 374:461–491. https://doi.org/10.1016/S0076-6879(03)74020-8
Ghislieri D, Turner NJ (2014) Biocatalytic approaches to the synthesis of enantiomerically pure chiral amines. Top Catal 57:284–300. https://doi.org/10.1007/s11244-013-0184-1
Grishin NV, Phillips MA, Goldsmith EJ (1995) Modeling of the spatial structure of eukaryotic ornithine decarboxylases. Protein Sci 4:1291–1304. https://doi.org/10.1002/pro.5560040705
Guo F, Berglund P (2017) Transaminase biocatalysis: optimization and application. Green Chem 19:333–360. https://doi.org/10.1039/c6gc02328b
Hekkelman ML, Te Beek TAH, Pettifer SR, Thorne D, Attwood TK, Vriend G (2010) WIWS: a protein structure bioinformatics web service collection. Nucleic Acids Res 38:W719–W723. https://doi.org/10.1093/nar/gkq453
Hult K, Berglund P (2007) Enzyme promiscuity: mechanism and applications. Trends Biotechnol 25:231–238. https://doi.org/10.1016/j.tibtech.2007.03.002
Humble MS, Engelmark Cassimjee K, Hakansson M, Kimbung YR, Walse B, Abedi V, Federsel H-J, Berglund P, Logan DT (2012) Crystal structures of the Chromobacterium Violaceum omega-transaminase reveal major structural rearrangements upon binding of coenzyme PLP. FEBS J 279:779–792. https://doi.org/10.1111/j.1742-4658.2012.08468.x
Inoue Y, Kuramitsu S, Inoue K, Kagamiyama H, Hiromi K, Tanase S, Morino Y (1989) Substitution of a lysyl residue for arginine 386 of Escherichia coli aspartate aminotransferase. J Biol Chem 264:9673–9681
Izumi Y, Sato K, Tani Y, Ogata K (1973) Purification of 7-keto-8-aminopelargonic acid-7, 8-diaminopelargonic acid aminotransferase, an enzyme involved in biotin biosynthesis, from Brevibacterium divaricatum. Agric Biol Chem 37:2683–2684. https://doi.org/10.1271/bbb1961.37.2683
Izumi Y, Sato K, Tani Y, Ogata K (1975) 7, 8-diaminopelargonic acid aminotransferase, an enzyme involved in biotin biosynthesis by microorganisms. Agric Biol Chem 39:175–181. https://doi.org/10.1271/bbb1961.39.175
Käck H, Sandmark J, Gibson K, Schneider G, Lindqvist Y (1999) Crystal structure of diaminopelargonic acid synthase: evolutionary relationships between pyridoxal-5′-phosphate-dependent enzymes. J Mol Biol 291:857–876. https://doi.org/10.1006/jmbi.1999.2997
Kaulmann U, Smithies K, Smith MEB, Hailes HC, Ward JM (2007) Substrate spectrum of ω-transaminase from Chromobacterium violaceum DSM30191 and its potential for biocatalysis. Enzyme Microb Tech 41:628–637. https://doi.org/10.1016/j.enzmictec.2007.05.011
Kirsch JF, Eichele G, Ford GC, Vincent MG, Jansonius JN, Gehring H, Christen P (1984) Mechanism of action of aspartate aminotransferase proposed on the basis of its spatial structure. J Mol Biol 174:497–525. https://doi.org/10.1016/0022-2836(84)90333-4
Krissinel E, Henrick K (2004) Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr D Biol Crystallogr 60:2256–2268. https://doi.org/10.1107/S0907444904026460
Krissinel E, Henrick K (2007) Inference of macromolecular assemblies from crystalline state. J Mol Biol 372:774–797. https://doi.org/10.1016/j.jmb.2007.05.022
Laskowski RA, Swindells MB (2011) LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model 51:2778–2786. https://doi.org/10.1021/ci200227u
Machell G (1960) The action of alkali on diacetyl. J Chem Soc 0:683–687. doi: https://doi.org/10.1039/JR9600000683
Mann S, Ploux O (2006) 7,8-Diaminoperlargonic acid aminotransferase from Mycobacterium tuberculosis, a potential therapeutic target. Characterization and inhibition studies. FEBS J 273:4778–4789. https://doi.org/10.1111/j.1742-4658.2006.05479.x
Mann S, Ploux O (2011) Pyridoxal-5′-phosphate-dependent enzymes involved in biotin biosynthesis: structure, reaction mechanism and inhibition. Biochim Biophys Acta 1814:1459–1466. https://doi.org/10.1016/j.bbapap.2010.12.004
Mann S, Colliandre L, Labesse G, Ploux O (2009) Inhibition of 7,8-diaminopelargonic acid aminotransferase from Mycobacterium tuberculosis by chiral and achiral analogs of its substrate: biological implications. Biochimie 91:826–834. https://doi.org/10.1016/j.biochi.2009.03.019
Midelfort KS, Kumar R, Han S, Karmilowicz MJ, McConnell K, Gehlhaar DK, Mistry A, Chang JS, Anderson M, Villalobos A, Minshull J, Govindarajan S, Wong JW (2013) Redesigning and characterizing the substrate specificity and activity of Vibrio fluvialis aminotransferase for the synthesis of imagabalin. Protein Eng Des Sel 26:25–33. https://doi.org/10.1093/protein/gzs065
Okamoto A, Nakai Y, Hayashi H, Hirotsu K, Kagamiyama H (1998) Crystal structures of Paracoccus denitrificans aromatic amino acid aminotransferase: a substrate recognition site constructed by rearrangement of hydrogen bond network. J Mol biol 280:443–461. https://doi.org/10.1006/jmbi.1998.1869
Padilla JE, Yeates TO (2003) A statistic for local intensity differences: robustness to anisotropy and pseudo-centering and utility for detecting twinning. Acta Crystallogr D Biol Crystallogr 59:1124–1130. https://doi.org/10.1107/S0907444903007947
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. https://doi.org/10.1002/jcc.20084
Pyrkov TV, Chugunov AO, Krylov N, Nolde DE, Efremov RG (2009) PLATINUM: a web tool for analysis of hydrophobic/hydrophilic organization of biomolecular complexes. Bioinformatics 25:1201–1202. https://doi.org/10.1093/bioinformatics/btp111
Rudat J, Brucher BR, Syldatk C (2012) Transaminases for the synthesis of enantiopure beta-amino acids. AMB Express 2:1–10. https://doi.org/10.1186/2191-0855-2-11
Salomon-Ferrer R, Götz AW, Poole D, Le Grand S, Walker RC (2013) Routine microsecond molecular dynamics simulations with AMBER on GPUs. 2. Explicit solvent particle mesh Ewald. J Chem Theory Comput 9:3878–3888. https://doi.org/10.1021/ct400314y
Sandmark J, Eliot AC, Famm K, Schneider G, Kirsch JF (2004) Conserved and nonconserved residues in the substrate binding site of 7,8-diaminopelargonic acid synthase from Escherichia coli are essential for catalysis. Biochemistry 43:1213–1222. https://doi.org/10.1021/bi0358059
Sayer C, Isupov MN, Westlake A, Littlechild JA (2013) Structural studies of Pseudomonas and Chromobacterium ω-aminotransferases provide insights into their differing substrate specificity. Acta Crystallogr D Biol Crystallogr 69:564–576. https://doi.org/10.1107/S0907444912051670
Schätzle S, Höhne M, Redestad E, Robins K, Bornscheuer UT (2009) Rapid and sensitive kinetic assay for characterization of omega-transaminases. Anal Chem 81:8244–8248. https://doi.org/10.1021/ac901640q
Schiroli D, Peracchi A (2015) A subfamily of PLP-dependent enzymes specialized in handling terminal amines, Biochim Biophys Acta. Proteins Proteomics 1854:1200–1211. https://doi.org/10.1016/j.bbapap.2015.02.023
Shin J-S, Yun H, Jang JW, Park I, Kim B-G (2003) Purification, characterization, and molecular cloning of a novel amine:pyruvate transaminase from Vibrio fluvialis JS17. Appl Microbiol Biot 61:463–471. https://doi.org/10.1007/s00253-003-1250-6
Shon M, Shanmugavel R, Shin G, Mathew S, Lee SH, Yun H (2014) Enzymatic synthesis of chiral γ-amino acids using ω-transaminase. Chem Commun 50:12680–12683. https://doi.org/10.1039/C3CC44864A
Simon RC, Grischek B, Zepeck F, Steinreiber A, Belaj F, Kroutil W (2012) Regio- and stereoselective monoamination of diketones without protecting groups. Angew Chem Int Ed Engl 51:6713–6716. https://doi.org/10.1002/anie.201202375
Søndergaard CR, Olsson MH, Rostkowski M, Jensen JH (2011) Improved treatment of ligands and coupling effects in empirical calculation and rationalization of pKa values. J Chem Theory Comput 7:2284–2295. https://doi.org/10.1021/ct200133y
Steffen-Munsberg F, Vickers C, Thontowi A, Schätzle S, Meinhardt T, Humble MS, Land H, Berglund P, Bornscheuer UT, Höhne M (2013) Revealing the structural basis of promiscuous amine transaminase activity. ChemCatChem 5:154–157. https://doi.org/10.1002/cctc.201200545
Steffen-Munsberg F, Vickers C, Kohls H, Land H, Mallin H, Nobili A, Skalden L, van den Bergh T, Joosten H-J, Berglund P, Höhne M, Bornscheuer UT (2015) Bioinformatic analysis of a PLP-dependent enzyme superfamily suitable for biocatalytic applications. Biotechnol Adv 33:566–604. https://doi.org/10.1016/j.biotechadv.2014.12.012
Steffen-Munsberg F, Matzel P, Sowa MA, Berglund P, Bornscheuer UT, Höhne M (2016) Bacillus anthracis ω-amino acid:pyruvate transaminase employs a different mechanism for dual substrate recognition than other amine transaminases. Appl Microbiol Biot 100:4511–4521. https://doi.org/10.1007/s00253-015-7275-9
Stoner GL, Eisenberg MA (1975) Biosynthesis of 7,8-diaminopelargonic acid from 7-keto-8-aminopelargonic acid and S-adenosyl-l-methionine. The kinetics of the reaction. J Biol Chem 250:4037–4043
Suplatov DA, Kopylov KE, Popova NN, Voevodin V, Švedas VK (2018) Mustguseal: a server for multiple structure-guided sequence alignment of protein families. Bioinformatics. https://doi.org/10.1093/bioinformatics/btx831
Tanaka H, Inaka K, Sugiyama S, Takahashi S, Sano S, Sato M, Yoshitomi S (2004) A simplified counter diffusion method combined with a 1D simulation program for optimizing crystallization conditions. J Synchrotron Radiat 11:45–48. https://doi.org/10.1107/S0909049503023446
Toney MD (2011) Controlling reaction specificity in pyridoxal phosphate enzymes. Biochim Biophys Acta, Proteins Proteomics 1814:1407–1418. https://doi.org/10.1016/j.bbapap.2011.05.019
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334
Vagin A, Lebedev A (2015) MoRDa, an automatic molecular replacement pipeline. Acta Crystallogr A 71:s19–s19. https://doi.org/10.1107/S2053273315099672
Van Arsdell SW, Perkins JB, Yocum RR, Luan L, Howitt CL, Chatterjee NP, Pero JG (2005) Removing a bottleneck in the Bacillus subtilis biotin pathway: bioA utilizes lysine rather than S-adenosylmethionine as the amino donor in the KAPA-to-DAPA reaction. Biotechnol Bioeng 91:75–83. https://doi.org/10.1002/bit.20488
Velick SF, Vavra J (1962) A kinetic and equilibrium analysis of the glutamic oxaloacetate transaminase mechanism. J Biol Chem 237:2109–2122
Watanabe N, Sakabe K, Sakabe N, Higashi T, Sasaki K, Aibara S, Morita Y, Yonaha K, Toyama S, Fukutani H (1989) Crystal structure analysis of ω-amino acid:pyruvate aminotransferase with a newly developed Weissenberg camera and an imaging plate using synchrotron radiation. J Biochem 105:1–3
Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AG, McCoy A, McNicholas SJ, Murshudov GN, Pannu NS, Potterton EA, Powell HR, Read RJ, Vagin A, Wilson KS (2011) Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr 67:235–242. https://doi.org/10.1107/S0907444910045749
Wybenga GG, Crismaru CG, Janssen DB, Dijkstra BW (2012) Structural determinants of the β-selectivity of a bacterial aminotransferase. J Biol Chem 287:28495–28502. https://doi.org/10.1074/jbc.M112.375238
Acknowledgements
The work including the protein production, biochemical characterization, and structural analysis was partially supported by the Russian Science Foundation (project 14-24-00172). The Russian Federal Space Agency supported the microgravity crystallization and data collection activities. Molecular docking calculations were supported by the Russian Foundation for Basic Research (Grant 16-34-60252). We thank the Federal Agency of Scientific Organizations for financial support of the protein purification. The authors are grateful to D.A. Suplatov, M.G. Khrenova and V.N. Novoseletsky for discussions and analysis of the docking results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 997 kb)
Rights and permissions
About this article
Cite this article
Bezsudnova, E.Y., Stekhanova, T.N., Popinako, A.V. et al. Diaminopelargonic acid transaminase from Psychrobacter cryohalolentis is active towards (S)-(-)-1-phenylethylamine, aldehydes and α-diketones. Appl Microbiol Biotechnol 102, 9621–9633 (2018). https://doi.org/10.1007/s00253-018-9310-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9310-0