Abstract
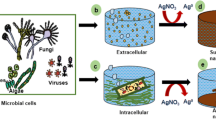
Sequential NanoFermentation (SNF) is a novel process which entails sparging microbially produced gas containing H2S from a primary reactor through a concentrated metal-acetate solution contained in a secondary reactor, thereby precipitating metallic sulfide nanoparticles (e.g., ZnS, CuS, or SnS). SNF holds an advantage over single reactor nanoparticle synthesis strategies, because it avoids exposing the microorganisms to high concentrations of toxic metal and sulfide ions. Also, by segregating the nanoparticle products from biological materials, SNF avoids coating nanoparticles with bioproducts that alter their desired properties. Herein, we report the properties of ZnS nanoparticles formed from SNF as compared with ones produced directly in a primary reactor (i.e., conventional NanoFermentation, or “CNF”), commercially available ZnS, and ZnS chemically synthesized by bubbling H2S gas through a Zn-acetate solution. The ZnS nanoparticles produced by SNF provided improved optical properties due to their smaller crystallite size, smaller overall particle sizes, reduced biotic surface coatings, and reduced structural defects. SNF still maintained the advantages of NanoFermentation technology over chemical synthesis including scalability, reproducibility, and lower hazardous waste burden.








Similar content being viewed by others
References
Akiba I (2002) Method for the production of anhydrous alkali metal sulfide and alkali metal sulfide solution. USA Patent 6,337,062
Eskelsen JR, Xu J, Chiu M, Moon J-W, Wilkins B, Graham DE, Gu B, Pierce EM (2018) Influence of structural defects on biomineralized ZnS nanoparticle dissolution: an in-situ electron microscopy study. Environ Sci Technol 52(3):1139–1149
Jang GG, Gresback RG, Ivanov IN, Meyer HM III, Kidder M, Phelps TJ, Graham DE, Moon J-W (2015a) Size tunable elemental copper nanoparticles: extracellular synthesis by thermoanaerobic bacteria and capping molecules. J Mater Chem C 3:644–650
Jang GG, Jacobs CB, Ivanov IN, Joshi PC, Meyer HM III, Kidder M, Armstrong BL, Datskos PG, Graham DE, Moon J-W (2015b) In situ capping for size control of monochalcogenide (ZnS, CdS and SnS) nanocrystals produced by anaerobic metal-reducing bacteria. Nanotechnol 26:325602
Kumar P, Saxena N, Singh F, Agarwal A (2012) Nanotwinning in CdS quantum dots. Physica B 407(17):3347–3351
Lindroos S, Kanniainen TML (1997) Growth of zinc sulfide thin films by the successive ionic layer adsorption and reaction (Silar) method on polyester substrates. Mater Res Bull 32(12):1631–1636
Madden AS, Swindle AL, Beazley MJ, Moon J-W, Ravel B, Phelps TJ (2012) Long-term solid-phase fate of co-precipitated U(VI)-Fe(III) following biological iron reduction by Thermoanaerobacter. Am Mineral 97(10):1641–1652
Moon J-W, Roh Y, Lauf RJ, Vali H, Yeary LW, Phelps TJ (2007a) Microbial preparation of metal-substituted magnetite nanoparticles. J Microbiol Methods 70(1):150–158
Moon J-W, Roh Y, Yeary LW, Lauf RJ, Rawn CJ, Love LJ, Phelps TJ (2007b) Microbial formation of lanthanide-substituted magnetites by Thermoanaerobacter sp. TOR-39. Extremophiles 11(6):859–867
Moon J-W, Rawn CJ, Rondinone AJ, Love LJ, Roh Y, Lauf RJ, Phelps TJ (2010a) Large-scale production of magnetic nanoparticles using bacterial fermentation. J Ind Microbiol Biotechnol 37(10):1023–1031
Moon J-W, Rawn CJ, Rondinone AJ, Wang W, Vali H, Yeary LW, Love LJ, Kirkham MJ, Gu B, Phelps TJ (2010b) Crystallite sizes and lattice parameters of nano-biomagnetite particles. J Nanosci Nanotechnol 10(12):8298–8306
Moon J-W, Ivanov IN, Duty CE, Love LJ, Rondinone AJ, Wang W, Li Y-L, Madden AS, Mosher JJ, Hu MZ, Suresh AK, Rawn CJ, Jung H, Lauf RJ, Phelps TJ (2013) Scalable economic extracellular synthesis of CdS nanostructured particles by a non-pathogenic thermophile. J Ind Microbiol Biotechnol 40(11):1263–1271
Moon J-W, Ivanov IN, Joshi PC, Armstrong BL, Wang W, Jung H, Rondinone AJ, Jellison G Jr, Meyer HM III, Jang GG, Meisner RA, Duty CE, Phelps TJ (2014) Scalable production of microbially-mediated ZnS nanoparticles and application to functional thin films. Acta Biomater 10(10):4474–4483
Moon J-W, Phelps TJ, Fitzgerald CL Jr, Lind RF, Elkins JG, Jang GG, Joshi PC, Kidder M, Armstrong BL, Watkins TR, Ivanov IN, Graham DE (2016) Manufacturing demonstration of microbially mediated zinc sulfide nanoparticles in pilot-plant scale reactors. Appl Microbiol Biotechnol 100(18):7921–7931
Murray CB, Norris DJ, Bawendi MG (1993) Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J Am Chem Soc 115(19):8706–8715
Nanda J, Sapra S, Sarma DD, Chandrasekharan N, Hodes G (2000) Size-selected zinc sulfide nanocrystallites: synthesis, structure, and optical studies. Chem Mater 12(4):1018–1024
Rodenbough PP, Song J, Walker D, Clark SM, Kalkan B, Chan SW (2015) Size dependent compressibility of nano-ceria: minimum near 33 nm. Appl Phys Lett 106(16):163101
Roh Y, Lauf RJ, McMillan AD, Zhang C, Rawn CJ, Bai J, Phelps TJ (2001) Microbial synthesis and the characterization of metal-substituted magnetites. Solid State Commun 118(10):529–534
Saunders JA (1998) Situ bioremediation of contaminated groundwater USA Patent US 5,833,855
Sim YJ, Hawang C-S (2016) Syntheses of the water-dispersible glycolic acid capped ZnS:Mn nanocrystals at different pH conditions, and their aggregation and luminescence quenching effects in aqueous solution. J Nanosci Nanotechnol 16(6):6281–6288
Umino H, Yamada N, Katagiri T, Shibuya H, Oguro S, Iwasaki N (2013) Reactor for synthesizing hydrogen sulfide apparatus for producing hydrogen sulfide, apparatus for producing sodium hydrogen sulfide, method for producing hydrogen sulfide, and method for producing sodium hydrogen sulfide. USA Patent US 8,551,442
Zhang H, Banfield JF (2009) Identification and growth mechanism of ZnS nanoparticles with mixed cubic and hexagonal stacking. J Phys Chem C 113(22):9681–9687
Zhang H, Chen B, Gilbert B, Banfield JF (2006) Kinetically controlled formation of a novel nanoparticulate ZnS with mixed cubic and hexagonal stacking. J Mater Chem 16(3):249–254
Zhang Y, Zhu F, Zhang J, Xia L (2008) Converting layered zinc acetate nanobelts to one-dimensional structured ZnO nanoparticle aggregates and their photocatalytic activity. Nanoscale Res Lett 3(6):201–204
Acknowledgements
The authors gratefully acknowledge support from the US Department of Energy (DOE), Office of Energy Efficiency & Renewable Energy’s Advanced Manufacturing Office, Low Temperature Material Synthesis Program (CPS 24762) of the Manufacturing Demonstration Facility. Part of this research was conducted at the Center for Nanophase Materials Sciences, which is sponsored by the ORNL Scientific User Facilities Division and DOE Office of Basic Research Sciences. EM characterization (JRE and EMP) was supported by the Office of Biological and Environmental Research (BER), Office of Science, DOE as part of the Mercury Science Focus Area at ORNL. The authors thank Deanne Brice for C&N analysis. FTIR work by M K was supported by the US DOE, Office of Science, Basic Energy Sciences under Award ERKCC96. ORNL is managed by UT-Battelle, LLC, for DOE under contract DE-AC05-00OR22725.
Disclaimers
Mention of brand name products does not constitute an endorsement by the U.S. Geological Survey. The data in this manuscript represent the entirety of the experimental work conducted.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Portions of this work may be subject to United States Application No. 62/359,356 filed on July 7, 2016 and WO2018009739A1 published on January 11, 2018 issued to inventors including coauthors JWM, TJP, RSO, DEG, INI, CBJ, GGJ, MKK, PCJ, and BLA. This article does not contain any studies with human participants or animals.
Additional information
This manuscript has been authored by UT-Battelle, LLC under Contract No. DE-AC05-00OR22725 with the U.S. Department of Energy. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. The Department of Energy will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Rights and permissions
About this article
Cite this article
Moon, JW., Eskelsen, J.R., Ivanov, I.N. et al. Improved ZnS nanoparticle properties through sequential NanoFermentation. Appl Microbiol Biotechnol 102, 8329–8339 (2018). https://doi.org/10.1007/s00253-018-9245-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9245-5