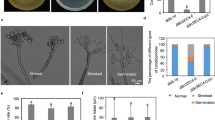
Abstract
Rab GTPases are the largest group of the small GTPases family, which play a pivotal role in the secretion of proteins. Arthrobotrys oligospora is a representative nematode-trapping fungus that can produce adhesive networks to capture nematodes. In this study, the roles of two Rab GTPases AoRab-7A and AoRab-2 were characterized by gene knockout in the fungus A. oligospora. The disruption of AoRab-7A hindered the mycelial growth in different media, the conidiation of ΔAoRab-7A transformants was almost abolished, and the transcription of four sporulation-related genes (AbaA, FluG, Hyp1, and VosA) was downregulated compared to the wild-type strain (WT). Furthermore, the tolerance of the ΔAoRab-7A mutants to sodium dodecyl sulfate (SDS) and H2O2 was also significantly reduced compared to the WT, and the transcription of several genes related to environmental resistance, such as genes for catalase and trehalose synthase, was downregulated. Similarly, the extracellular proteolytic activity was decreased. Importantly, the ΔAoRab-7A mutants were unable to produce traps and capture nematodes. However, the disruption of gene AoRab-2 only affected the conidiation slightly but non-significantly, while other phenotypic traits were unaffected. Moreover, the gene AoRab-7A was also involved in the autophagy induced by nitrogen deprivation in A. oligospora. Our results revealed for the first time that the Rab GTPases are involved in the regulation of mycelial growth, conidiation, trap formation, stress resistance, and pathogenicity in the nematode-trapping fungus A. oligospora.







Similar content being viewed by others
References
Abenza JF, Galindo A, Pinar M, Pantazopoulou A, de los Ríos V, Peñalva MA (2012) Endosomal maturation by Rab conversion in Aspergillus nidulans is coupled to dynein-mediated basipetal movement. Mol Biol Cell 23:1889–1901. https://doi.org/10.1091/mbc.E11-11-0925
Ahrén D, Tholander M, Fekete C, Rajashekar B, Friman E, Johansson T, Tunlid A (2005) Comparison of gene expression in trap cells and vegetative hyphae of the nematophagous fungus Monacrosporium haptotylum. Microbiology 151:789–803. https://doi.org/10.1099/mic.0.27485-0
Angelova MB, Pashova SB, Spasova BK, Vassilev SV, Slokoska LS (2005) Oxidative stress response of filamentous fungi induced by hydrogen peroxide and paraquat. Mycol Res 109:150–158. https://doi.org/10.1017/S0953756204001352
Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B (2000) Rab7: a key to lysosome biogenesis. Mol Biol Cell 11:467–480. https://doi.org/10.1091/mbc.11.2.467
Chen YL, Gao Y, Zhang KQ, Zou CG (2013) Autophagy is required for trap formation in the nematode-trapping fungus Arthrobotrys oligospora. Environ Microbiol Rep 5:511–517. https://doi.org/10.1111/1758-2229.12054
Cheung AY, Chen CYH, Glaven RH, De Graaf BH, Vidali L, Hepler PK, Wu HM (2002) Rab2 GTPase regulates vesicle trafficking between the endoplasmic reticulum and the Golgi bodies and is important to pollen tube growth. Plant Cell 14:945–962. https://doi.org/10.1105/tpc.000836
Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P (1992) Multifunctional yeast high-copy-number shuttle vectors. Gene 110:119–122. https://doi.org/10.1016/0378-1119(92)90454-W
Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, Weiss R, Borkovich KA, Dunlap JC (2006) A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci U S A 103:10352–10357. https://doi.org/10.1073/pnas.0601456103
Conesa A, Punt PJ, van Luijk N, van den Hondel CA (2001) The secretion pathway in filamentous fungi: a biotechnological view. Fungal Genet Biol 33:155–171. https://doi.org/10.1006/fgbi.2001.1276
Contento AL, Xiong Y, Bassham DC (2005) Visualization of autophagy in Arabidopsis using the fluorescent dye monodansylcadaverine and a GFP-AtATG8e fusion protein. Plant J 42:598–608. https://doi.org/10.1111/j.1365-313X.2005.02396.x
Espino JJ, Gutiérrez-Sánchez G, Brito N, Shah P, Orlando R, González C (2010) The Botrytis cinerea early secretome. Proteomics 10:3020–3034. https://doi.org/10.1002/pmic.201000037
Farnesi LC, Menna-Barreto RFS, Martins AJ, Valle D, Rezende GL (2015) Physical features and chitin content of eggs from the mosquito vectors Aedes aegypti, Anopheles aquasalis and Culex quinquefasciatus: connection with distinct levels of resistance to desiccation. J Insect Physiol 83:43–52. https://doi.org/10.1016/j.jinsphys.2015.10.006
Fekete C, Tholander M, Rajashekar B, Ahrén D, Friman E, Johansson T, Tunlid A (2008) Paralysis of nematodes: shifts in the transcriptome of the nematode-trapping fungus Monacrosporium haptotylum during infection of Caenorhabditis elegans. Environ Microbiol 10:364–375. https://doi.org/10.1111/j.1462-2920.2007.01457.x
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins M, Appel R, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In: Walker J (ed) The proteomics protocols handbook. Humana Press, Totowa, pp 571–607. https://doi.org/10.1385/1-59259-890-0:571
Grant CM (2001) Role of the glutathione/glutaredoxin and thioredoxin systems in yeast growth and response to stress conditions. Mol Microbiol 39:533–541. https://doi.org/10.1046/j.1365-2958.2001.02283.x
Gray NF (1984) Ecology of nematophagous fungi: comparison of the soil sprinkling method with the Baermann funnel technique in the isolation of endoparasites. Soil Biol Biochem 16:81–83. https://doi.org/10.1016/0038-0717(84)90131-7
Jiang D, Zhou J, Bai G, Xing X, Tang L, Yang X, Li J, Zhang KQ, Yang JK (2017) Random mutagenesis analysis and identification of a novel C2H2-type transcription factor from the nematode-trapping fungus Arthrobotrys oligospora. Sci Rep 7:5640. https://doi.org/10.1038/s41598-017-06075-5
Jones P, Binns D, Chang HY, Fraser M, Li W, McAnulla C, Mcwillian H, Maslen J, Mitchell A, Nuka G, Pesseat S (2014) InterProScan 5: genome-scale protein function classification. Bioinformatics 30:1236–1240. https://doi.org/10.1093/bioinformatics/btu031
Kikuma T, Arioka M, Kitamoto K (2007) Autophagy during conidiation and conidial germination in filamentous fungi. Autophagy 3:128–129. https://doi.org/10.4161/auto.3560
Krijgsheld P, Bleichrodt RV, Van Veluw GJ, Wang F, Müller WH, Dijksterhuis J, Wösten HAB (2013) Development in Aspergillus. Stud Mycol 74:1–29. https://doi.org/10.3114/sim0006
Li Y, Hyde KD, Jeewon R, Cai L, Vijaykrishna D, Zhang K (2005) Phylogenetics and evolution of nematode-trapping fungi (Orbiliales) estimated from nuclear and protein coding genes. Mycologia 97:1034–1046. https://doi.org/10.1080/15572536.2006.11832753
Li J, Gu F, Wu R, Yang J, Zhang KQ (2017) Phylogenomic evolutionary surveys of subtilase superfamily genes in fungi. Sci Rep 7:45456. https://doi.org/10.1038/srep45456
Liu Q, Ying SH, Feng MG (2011) Characterization of Beauveria bassiana neutral trehalase (BbNTH1) and recognition of crucial stress-responsive elements to control its expression in response to multiple stresses. Microbiol Res 166:282–293. https://doi.org/10.1016/j.micres.2010.04.001
Liu K, Zhang W, Lai Y, Xiang M, Wang X, Zhang X, Liu X (2014) Drechslerella stenobrocha genome illustrates the mechanism of constricting rings and the origin of nematode predation in fungi. BMC Genomics 15:114. https://doi.org/10.1186/1471-2164-15-114
Liu XH, Chen SM, Gao HM, Ning GA, Shi HB, Wang Y, Dong B, Qi YY, Zhang DM, Lu GD, Wang DH, Zhou J, Lin FC (2015) The small GTPase MoYpt7 is required for membrane fusion in autophagy and pathogenicity of Magnaporthe oryzae. Environ Microbiol 17:4495–4510. https://doi.org/10.1111/1462-2920.12903
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Luo X, Keyhani NO, Yu X, He Z, Luo Z, Pei Y, Zhang Y (2012) The MAP kinase Bbslt2 controls growth, conidiation, cell wall integrity, and virulence in the insect pathogenic fungus Beauveria bassiana. Fungal Genet Biol 49:544–555. https://doi.org/10.1016/j.fgb.2012.05.002
Meerupati T, Andersson KM, Friman E, Kumar D, Tunlid A, Ahrén D (2013) Genomic mechanisms accounting for the adaptation to parasitism in nematode-trapping fungi. PLoS Genet 9:e1003909. https://doi.org/10.1371/journal.pgen.1003909
Mitin N, Rossman KL, Der CJ (2005) Signaling interplay in Ras superfamily function. Curr Biol 15:R563–R574. https://doi.org/10.1016/j.cub.2005.07.010
Nordbring-Hertz B, Jansson H-B, Tunlid A (2011) Nematophagous fungi. In: Encyclopedia of life sciences. John Wiley & Sons, Chichester, pp 1–13. https://doi.org/10.1002/9780470015902.a0000374.pub3
Novick P, Zerial M (1997) The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol 9:496–504. https://doi.org/10.1016/S0955-0674(97)80025-7
Pantazopoulou A, Peñalva MA (2011) Characterization of Aspergillus nidulans RabC/Rab6. Traffic 12:386–406. https://doi.org/10.1111/j.1600-0854.2011.01164.x
Park HS, Yu JH (2012) Genetic control of asexual sporulation in filamentous fungi. Curr Opin Microbiol 15:669–677. https://doi.org/10.1016/j.mib.2012.09.006
Powers-Fletcher MV, Feng X, Krishnan K, Askew DS (2013) Deletion of the sec4 homolog srgA from Aspergillus fumigatus is associated with an impaired stress response, attenuated virulence and phenotypic heterogeneity. PLoS One 8:e66741. https://doi.org/10.1371/journal.pone.0066741
Punt PJ, Seiboth B, Weenink XO, van Zeijl C, Lenders M, Konetschny C, Ram AFJ, Montijn R, Kubicek CP, van den Hondel CAMJJ (2001) Identification and characterization of a family of secretion-related small GTPase-encoding genes from the filamentous fungus Aspergillus niger: a putative SEC4 homologue is not essential for growth. Mol Microbiol 41:513–525. https://doi.org/10.1046/j.1365-2958.2001.02541.x
Rajan N, Agarwal P, Patel K, Sanadhya P, Khedia J, Agarwal PK (2015) Molecular characterization and identification of target protein of an important vesicle trafficking gene AlRab7 from a salt excreting halophyte Aeluropus lagopoides. DNA Cell Biol 34:83–91. https://doi.org/10.1089/dna.2014.2592
Segev N, Mulholland J, Botstein D (1988) The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell 52:915–924. https://doi.org/10.1016/0092-8674(88)90433-3
Sewall TC, Mims CW, Timberlake WE (1990) AbaA controls phialide differentiation in Aspergillus nidulans. Plant Cell 2:731–739. https://doi.org/10.2307/3869172
Siriputthaiwan P, Jauneau A, Herbert C, Garcin D, Dumas B (2005) Functional analysis of CLPT1, a Rab/GTPase required for protein secretion and pathogenesis in the plant fungal pathogen Colletotrichum lindemuthianum. J Cell Sci 118:323–329. https://doi.org/10.1242/jcs.01616
Staben C, Jensen B, Singer M, Pollock J, Schechtman M, Kinsey J, Selker E (1989) Use of a bacterial hygromycin B resistance gene as a dominant selectable marker in Neurospora crassa transformation. Fungal Genet Rep 36:79–81. https://doi.org/10.4148/1941-4765.1519
Stenmark H, Olkkonen VM (2001) The Rab GTPase family. Genome Biol 2:3007–3001. https://doi.org/10.1186/gb-2001-2-5-reviews3007
Su H, Zhao Y, Zhou J, Feng H, Jiang D, Zhang KQ, Yang JQ (2017) Trapping devices of nematode-trapping fungi: formation, evolution, and genomic perspectives. Biol Rev 92:357–368. https://doi.org/10.1111/brv.12233
Takai Y, Sasaki T, Matozaki T (2001) Small GTP-binding proteins. Physiol Rev 81:153–208. https://doi.org/10.1016/S0074-7696(08)61861-6
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. https://doi.org/10.1093/molbev/msr121
Tao L, Yu JH (2011) AbaA and WetA govern distinct stages of Aspergillus fumigatus development. Microbiology 157:313–326. https://doi.org/10.1099/mic.0.044271-0
Thön M, Al-Abdallah Q, Hortschansky P, Brakhage AA (2007) The thioredoxin system of the filamentous fungus Aspergillus nidulans: impact on development and oxidative stress response. J Biol Chem 282:27259–27269. https://doi.org/10.1074/jbc.M704298200
Tian S, Zhang Z, Qin G (2014) Function of Rab GTPases in regulating the development, protein secretion and virulence of fungi. In: Prusky D, Gullino ML (eds) Post-harvest pathology. Springer, Cham, pp 3–9. https://doi.org/10.1007/978-3-319-07701-7_1
Tunlid A, Rosén S, Ek BO, Rask L (1994) Purification and characterization of an extracellular serine protease from the nematode-trapping fungus Arthrobotrys oligospora. Microbiology 140:1687–1695. https://doi.org/10.1099/13500872-140-7-1687
Tunlid A, Åhman J, Oliver RP (1999) Transformation of the nematode-trapping fungus Arthrobotrys oligospora. FEMS Microbiol Lett 173:111–116. https://doi.org/10.1111/j.1574-6968.1999.tb13491.x
Wang XX, He PH, Feng MG, Ying SH (2014) BbSNF1 contributes to cell differentiation, extracellular acidification, and virulence in Beauveria bassiana, a filamentous entomopathogenic fungus. Appl Microbiol Biotechnol 98:8657–8673. https://doi.org/10.1007/s00253-014-5907-0
Winston F, Dollard C, Ricupero-Hovasse SL (1995) Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11:53–55. https://doi.org/10.1002/yea.320110107
Yang JK, Huang X, Tian B, Wang M, Niu Q, Zhang KQ (2005) Isolation and characterization of a serine protease from the nematophagous fungus, Lecanicillium psalliotae, displaying nematicidal activity. Biotechnol Lett 27:1123–1128. https://doi.org/10.1007/s10529-005-8461-0
Yang JK, Wang L, Ji XL, Feng Y, Li XM, Zou CG, Xu JP, Ren Y, Mi QL, Wu JL, Liu SQ, Liu Y, Huang XW, Niu XM, Li J, Liang LM, Luo YL, Ji KF, Zhou W, Yu ZF, Li GH, Liu YJ, Li L, Qiao M, Feng L, Zhang KQ (2011) Genomic and proteomic analyses of the fungus Arthrobotrys oligospora provide insights into nematode-trap formation. PLoS Pathog 7:e1002179. https://doi.org/10.1371/journal.ppat.1002179
Yang E, Xu L, Yang Y, Zhang X, Xiang M, Wang C, An ZQ, Liu XZ (2012) Origin and evolution of carnivorism in the Ascomycota (fungi). Proc Natl Acad Sci U S A 109:10960–10965. https://doi.org/10.1073/pnas.1120915109
Yang JK, Liang L, Li J, Zhang KQ (2013) Nematicidal enzymes from microorganisms and their applications. Appl Microbiol Biotechnol 97:7081–7095. https://doi.org/10.1007/s00253-013-5045-0
Zhang L, Yang JK, Niu Q, Zhao X, Ye F, Liang L, Zhang KQ (2008) Investigation on the infection mechanism of the fungus Clonostachys rosea against nematodes using the green fluorescent protein. Appl Microbiol Biotechnol 78:983–990. https://doi.org/10.1007/s00253-008-1392-7
Zhang Z, Qin G, Li B, Tian S (2014) Knocking out Bcsas1 in Botrytis cinerea impacts growth, development, and secretion of extracellular proteins, which decreases virulence. Mol Plant-Microbe Interact 27:590–600. https://doi.org/10.1094/MPMI-10-13-0314-R
Zhao ML, Mo MH, Zhang KQ (2004) Characterization of a neutral serine protease and its full-length cDNA from the nematode-trapping fungus Arthrobotrys oligospora. Mycologia 96:16–22. https://doi.org/10.1080/15572536.2005.11832991
Zhao XY, Wang Y, Zhao Y, Huang Y, Zhang KQ, Yang JK (2014) Malate synthase gene AoMls in the nematode-trapping fungus Arthrobotrys oligospora contributes to conidiation, trap formation, and pathogenicity. Appl Microbiol Biotechnol 98:2555–2563. https://doi.org/10.1007/s00253-013-5432-6
Zheng H, Zheng W, Wu C, Yang J, Xi Y, Xie Q, Zhao X, Deng XL, Lu GD, Li GP, Ebbole D, Zhou J, Wang ZH (2015) Rab GTPases are essential for membrane trafficking-dependent growth and pathogenicity in Fusarium graminearum. Environ Microbiol 17:4580–4599. https://doi.org/10.1111/1462-2920.12982
Acknowledgements
We are grateful to Prof. Jianping Xu of the Dept. of Biology, McMaster University, for his valuable comments and critical discussions.
Funding
The research described here is jointly supported by the NSFC-Yunnan Joint Fund (U1402265), the National Basic Research Program of China (2013CB127503), the National Natural Science Foundation of China (approved nos. 31272093, 31360019, and 31560025), the Program for Excellent Young Talents of Yunnan University (to Jinkui Yang), and the General Program of the Applied Basic Research Programs of Yunnan Province (approval no. 2016FB044).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 1662 kb).
Rights and permissions
About this article
Cite this article
Yang, X., Ma, N., Yang, L. et al. Two Rab GTPases play different roles in conidiation, trap formation, stress resistance, and virulence in the nematode-trapping fungus Arthrobotrys oligospora. Appl Microbiol Biotechnol 102, 4601–4613 (2018). https://doi.org/10.1007/s00253-018-8929-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8929-1