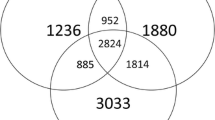
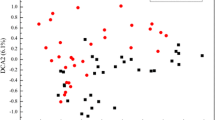
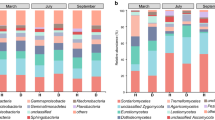
Abstract
Bacterial wilt, caused by Ralstonia solanacearum, occurs occasionally during tobacco planting and potentially brings huge economic losses in affected areas. Soil microbes in different management stages play important roles in influencing bacterial wilt incidence. Studies have focused on the impacts of species diversity and composition during cropping periods on disease morbidity; however, the effects of the soil bacterial biomass, species diversity, species succession, and population interactions on morbidity remain unclear during non-cropping periods. In this study, we explored the soil bacterial communities in the non-cropping winter fallow (WF) and cropping late growing (LG) periods under consecutive monoculture systems using 16S ribosomal RNA gene sequencing and qPCR and further analyzed their effects on tobacco bacterial wilt incidence. We found that the bacterial communities in the WF period were significantly different from those in the LG period based on detrended correspondence analysis and dissimilarity tests. Crop morbidity was significantly related to bacterial community structure and to the presence of some genera during WF and LG periods. These genera, such as Arthrobacter, Pseudomonas, Acidobacteria GP6, and Pasteuria, may be potential biological control agents for bacterial wilt. Further analysis indicated that low soil bacterial diversity during the WF period, decrease of bacterial interactions from the WF to LG periods, and low soil biomass during the LG period all have the potential to increase morbidity. In conclusion, an increase of soil bacterial diversity and control of some bacterial abundances in the WF period might be an effective approach in controlling bacterial wilt incidence.







Similar content being viewed by others
References
Ahimou F, Deleu MJP (2000) Surfactin and iturin A effects on Bacillus subtilis surface hydrophobicity. Enzym Microb Technol 27(10):749–754. https://doi.org/10.1016/S0141-0229(00)00295-7
Barberán A, Ramirez KS, Leff JW, Bradford MA, Wall DH, Fierer N (2014) Why are some microbes more ubiquitous than others? Predicting the habitat breadth of soil bacteria. Ecol Lett 17(7):794–802. https://doi.org/10.1111/ele.12282
Berkovitch YA (1996) Instrumentation for plant health and growth in space. Adv Space Res 18(4–5):157–162. https://doi.org/10.1016/0273-1177(95)00872-C
Bramley R, Ellis N, Nable RO, Garside AL (1996) Changes in soil chemical properties under long-term sugar cane monoculture and their possible role in sugar yield decline. Aust J Soil Res 34(6):967–984. https://doi.org/10.1071/SR9960967
Cui P, Fan F, Yin C, Song A, Huang P, Tang Y, Zhu P, Peng C, Li T, Wakelin SA, Liang Y (2016) Long-term organic and inorganic fertilization alters temperature sensitivity of potential N2O emissions and associated microbes. Soil Biol Biochem 93(131–141. https://doi.org/10.1016/j.soilbio.2015.11.005
Defoirdt T, Boon N, Sorgeloos P, Verstraete W, Bossier P (2007) Alternatives to antibiotics to control bacterial infections: luminescent vibriosis in aquaculture as an example. Trends Biotechnol 25(10):472–479. https://doi.org/10.1128/AEM.01610-13
Deng Y, He Z, Xu M, Qin Y, Van Nostrand JD, Wu L, Roe BA, Wiley G, Hobbie SE, Reich PB (2012a) Elevated carbon dioxide alters the structure of soil microbial communities. Appl Environ Microb 78(8):2991–2995. https://doi.org/10.1128/AEM.06924-11
Deng Y, Jiang YH, Yang Y, He Z, Luo F, Zhou J (2012b) Molecular ecological network analyses. BMC Bioinformatics 13(1):113. https://doi.org/10.1186/1471-2105-13-113
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26(19):2460–2461. https://doi.org/10.1093/bioinformatics/btq461
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27(16):2194–2200. https://doi.org/10.1093/bioinformatics/btr381
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. P Nat Acad Sci USA 103(3):626–631. https://doi.org/10.1073/pnas.0507535103
Franke-Whittle IH, Manici LM, Insam H, Stres B (2015) Rhizosphere bacteria and fungi associated with plant growth in soils of three replanted apple orchards. Plant Soil 395(1):317–333. https://doi.org/10.1007/s11104-015-2562-x
Franklin RB, Mills AL (2006) Structural and functional responses of a sewage microbial community to dilution-induced reductions in diversity. Microbial Ecol 52(2):280–288. https://doi.org/10.1007/s00248-006-9033-0
Fu L, Penton CR, Ruan Y, Shen Z, Xue C, Li R, Shen Q (2017) Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol Biochem 104:39–48. https://doi.org/10.1016/j.soilbio.2016.10.008
Garrido P, Gonzalez-Toril E, Garcia-Moyano A, Moreno-Paz M, Amils R, Parro V (2008) An oligonucleotide prokaryotic acidophile microarray: its validation and its use to monitor seasonal variations in extreme acidic environments with total environmental RNA. Environ Microbiol 10(4):836–850. https://doi.org/10.1111/j.1462-2920.2008.01477.x
Granér G, Persson P, Meijer J, Alström S (2003) A study on microbial diversity in different cultivars of Brassica napus in relation to its wilt pathogen, Verticillium longisporum. FEMS Microbiol Lett 224(2):269–276. https://doi.org/10.1016/S0378-1097(03)00449-X
Hai NV (2015) The use of probiotics in aquaculture. J Appl Microbiol 119(4):917–935. https://doi.org/10.1111/jam.12886
Handelsman J, Nesmith WC, Raffel SJ (1991) Microassay for biological and chemical control of infection of tobacco by Phytophthora parasitica var. nicotianae. Curr Microbiol 22(5):317–319. https://doi.org/10.1007/BF02091961
Handelsman J, Raffel S, Mester EH, Wunderlich L, Grau CR (1990) Biological control of damping-off of alfalfa seedlings with Bacillus cereus UW85. Appl Environ Microb 56(3):713–718
Hu Y, Wu K, Liu N, Chen H, Jia X (2004) Studies on microbial population dynamics in the cucumber rhizospheres at different developmental stages. Sci Agri Sinica 37(10):1521–1526
Huo Q, Zhang S, Wang R (2007) Advance and control of tobacco bacterial wilt disease. Chin Agri Sci Bull:364–368
Ji G, Wei L, He Y, Wu Y, Bai X (2008) Biological control of rice bacterial blight by Lysobacter antibioticus strain 13-1. Biol Control 45(3):288–296. https://doi.org/10.1016/j.biocontrol.2008.01.004
Johnson PTJ, Preston DL, Hoverman JT, Henderson JS, Paull SH, Richgels KLD, Redmond MD (2012) Species diversity reduces parasite infection through cross-generational effects on host abundance. Ecology 93(1):56–64. https://doi.org/10.1890/11-0636.1
Kailasapathy K (2002) Microencapsulation of probiotic bacteria: technology and potential applications. Curr Iss Intest Microbiol 3(2):39–48
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L (2008) Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquaculture 274(1):1–14. https://doi.org/10.1016/j.aquaculture.2007.11.019
Kloepper JW, Rodríguez-Kábana R, Zehnder AW, Murphy JF, Sikora E, Fernández C (1999) Plant root-bacterial interactions in biological control of soilborne diseases and potential extension to systemic and foliar diseases. Australas Plant Path 28(1):21–26. https://doi.org/10.1071/AP99003
Kloepper JW, Leong J, Teintze M, Schroth MN (1980) Pseudomonas siderophores: a mechanism explaining disease-suppressive soils. Curr Microbiol 4(5):317–320. https://doi.org/10.1007/BF02602840
Kyselková M, Kopecký J, Frapolli M, Défago G, Ságová-Marečková M, Grundmann GL, Moënne-Loccoz Y (2009) Comparison of rhizobacterial community composition in soil suppressive or conducive to tobacco black root rot disease. ISME J 3(10):1127–1138. https://doi.org/10.1038/ismej.2009.61
Lacroix C, Jolles A, Seabloom EW, Power AG, Mitchell CE, Borer ET (2013) Non-random biodiversity loss underlies predictable increases in viral disease prevalence. J Roy Soc Int 11(92):20130947. https://doi.org/10.1098/rsif.2013.0947
Leifert C, Li H, Chidburee S, Hampson S, Workman S, Sigee D, Epton HAS, Harbour A (1995) Antibiotic production and biocontrol activity by Bacillus subtilis CL27 and Bacillus pumilus CL45. J Appl Bacteriol 78(2):97–108. https://doi.org/10.1111/j.1365-2672.1995.tb02829.x
Liu J, Sui Y, Yu Z, Yao Q, Shi Y, Chu H, Jin J, Liu X, Wang G (2016) Diversity and distribution patterns of acidobacterial communities in the black soil zone of northeast China. Soil Biol Biochem 95:212–222. https://doi.org/10.1016/j.soilbio.2015.12.021
Lou L, Qian G, Xie Y, Hang J, Chen H, Zaleta-Rivera K, Li Y, Shen Y, Dussault PH, Liu F (2011) Biosynthesis of HSAF, a tetramic acid-containing macrolactam from Lysobacter enzymogenes. J Am Chem Soc 133(4):643–645. https://doi.org/10.1021/ja105732c
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Ann Rev Microbiol 63(63):541–556. https://doi.org/10.1146/annurev.micro.62.081307.162918
Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, Dow M, Verdier V, Beer SV, Machado MA (2012) Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol 13(6):614–629. https://doi.org/10.1111/j.1364-3703.2012.00804.x
Matos A, Kerkhof L, Garland JL (2005) Effects of microbial community diversity on the survival of Pseudomonas aeruginosa in the wheat rhizosphere. Microbial Ecol 49(2):257–264. https://doi.org/10.1007/s00248-004-0179-3
Mazurier S, Corberand T, Lemanceau P, Raaijmakers JM (2009) Phenazine antibiotics produced by fluorescent pseudomonads contribute to natural soil suppressiveness to Fusarium wilt. ISME J 3(8):977–991. https://doi.org/10.1038/ismej.2009.33
Mengesha WK, Powell SM, Evans KJ, Barry KM (2017) Diverse microbial communities in non-aerated compost teas suppress bacterial wilt. W J Microb Biot 33(3):49. https://doi.org/10.1007/s11274-017-2212-y
Moyne AL, Cleveland TE, Tuzun S (2004) Molecular characterization and analysis of the operon encoding the antifungal lipopeptide bacillomycin D. FEMS Microbiol Lett 234(1):43–49. https://doi.org/10.1111/j.1574-6968.2004.tb09511.x
Niu J, Chao J, Xiao Y, Chen W, Zhang C, Liu X, Rang Z, Yin H, Dai L (2017) Insight into the effects of different cropping systems on soil bacterial community and tobacco bacterial wilt rate. J Basic Microbiol 57(1):3–11. https://doi.org/10.1002/jobm.201600222
Pal KK, Gardener MS (2006) Biological control of plant pathogens. Plant Health Instructor 202:147
Schmidt HP, Kammann C, Niggli C, Evangelou MWH, Mackie KA, Abiven S (2014) Biochar and biochar-compost as soil amendments to a vineyard soil: influences on plant growth, nutrient uptake, plant health and grape quality. Agri Ecosyst Environ 191(117–123. https://doi.org/10.1016/j.agee.2014.04.001
Sharifi-Tehrani A, Zala M, Natsch A, Moënne-Loccoz Y, Défago G (1998) Biocontrol of soil-borne fungal plant diseases by 2,4-diacetylphloroglucinol-producing fluorescent pseudomonads with different restriction profiles of amplified 16S rDNA. Eur J Plant Pathol 104(7):631–643. https://doi.org/10.1023/A:1008672104562
She S, Niu J, Zhang C, Xiao Y, Chen W, Dai L, Liu X, Yin H (2016) Significant relationship between soil bacterial community structure and incidence of bacterial wilt disease under continuous cropping system. Arch Microbiol 199(2):267–275. https://doi.org/10.1007/s00203-016-1301-x
Shen Z, Ruan Y, Xue C, Zhong S, Li R, Shen Q (2015) Soils naturally suppressive to banana Fusarium wilt disease harbor unique bacterial communities. Plant Soil 393(1):1–13. https://doi.org/10.1007/s11104-015-2474-9
Shi S, O Callaghan M, Jones EE, Richardson AE, Walter C, Stewart A, Condron L (2012) Investigation of organic anions in tree root exudates and rhizosphere microbial communities using in situ and destructive sampling techniques. Plant Soil 359(359):149–163. https://doi.org/10.1007/s11104-012-1198-3
Shiomi Y, Nishiyama M, Onizuka T, Marumoto T (1999) Comparison of bacterial community structures in the rhizoplane of tomato plants grown in soils suppressive and conducive towards bacterial wilt. Appl Environ Microb 65(9):3996–4001
Silo-Suh LA, Lethbridge BJ, Raffel SJ, He H, Clardy J, Handelsman J (1994) Biological activities of two fungistatic antibiotics produced by Bacillus cereus UW85. Appl Environ Microb 60(6):2023–2030
Smith KP, Havey MJ, Handelsman J (1993) Suppression of cottony leak of cucumber with Bacillus cereus strain UW85. Plant Dis 77(2):139. https://doi.org/10.1094/PD-77-0139
Spence C, Alff E, Johnson C, Ramos C, Donofrio N, Sundaresan V, Bais H (2014) Natural rice rhizospheric microbes suppress rice blast infections. BMC Plant Biol 14(1):130. https://doi.org/10.1186/1471-2229-14-130
van Elsas JD, Jansson J, Trevors JT (1997) Modern soil microbiology. Marcel Dekker, New York
van Elsas JD, Garbeva P, Salles J (2002) Effects of agronomical measures on the microbial diversity of soils as related to the suppression of soil-borne plant pathogens. Biodegradation 13(1):29–40
van Elsas JD, Salles JF (2012) Microbial diversity determines the invasion of soil by a bacterial pathogen. P Nat Acad Sci USA 109(4):1159–1164. https://doi.org/10.1073/pnas.1109326109
Voisard C, Keel C, Haas D, Dèfago G (1989) Cyanide production by Pseudomonas fluorescens helps suppress black root rot of tobacco under gnotobiotic conditions. EMBO J 8(2):351–358
Wagg C, Bender SF, Widmer F, van der Heijden MGA (2014) Soil biodiversity and soil community composition determine ecosystem multifunctionality. P Nat Acad Sci 111(14):5266–5270. https://doi.org/10.1073/pnas.1320054111
Ward NL, Challacombe JF, Janssen PH, Bernard H, Coutinho PM, Martin W, Gary X, Haft DH, Michelle S, Jonathan B (2009) Three genomes from the phylum Acidobacteria provide insight into the lifestyles of these microorganisms in soils. Appl Environ Microb 75(7):2046–2056. https://doi.org/10.1128/AEM.02294-08
Wu L, Wen C, Qin Y, Yin H, Tu Q, Nostrand JDV, Yuan T, Yuan M, Ye D, Zhou J (2015) Phasing amplicon sequencing on Illumina Miseq for robust environmental microbial community analysis. BMC Microbiol 15(1):1–12. https://doi.org/10.1186/s12866-015-0450-4
Yang H, Niu J, Tao J, Gu Y, Zhang C, She S, Chen W, Yang H, Yin H (2016) The impacts of different green manure on soil microbial communities and crop health. Preprints. doi: https://doi.org/10.20944/Preprints 2016:2016090056
Yin C, Hulbert SH, Schroeder KL (2013) Role of bacterial communities in the natural suppression of rhizoctonia solani bare patch disease of wheat (Triticum aestivum L.) Appl Environ Microb 79(23):7428–7438. https://doi.org/10.1128/AEM.01610-13
Yin H, Niu J, Ren Y, Cong J, Zhang X, Fan F, Xiao Y, Zhang X, Deng J, Xie M (2015) An integrated insight into the response of sedimentary microbial communities to heavy metal contamination. Sci Rep 5(1):93–102. https://doi.org/10.1038/srep14266
Yoshiko I, Masaya N, Shigeto O, Keishi S (2006) Rhizobacterial community-level, sole carbon source utilization pattern affects the delay in the bacterial wilt of tomato grown in rhizobacterial community model system. Appl Soil Ecol 34(1):27–32. https://doi.org/10.1016/j.apsoil.2005.12.003
Zhan F, Lu Y, Guan G (2005) Community structures of microorganisms and their dynamics in the rhizosphere of flue-cured tobacco. Acta Pedol Sin 42(3):488–494
Zhou J, Deng Y, Luo F, He Z, Tu Q, Zhi X (2010) Functional molecular ecological networks. MBio 1(4):e00169-10–e00169-19. https://doi.org/10.1128/mBio.00169-10
Zhou J, Deng Y, Zhang P, Xue K, Liang Y, Van Nostrand JD, Yang Y, He Z, Wu L, Stahl DA (2014) Stochasticity, succession, and environmental perturbations in a fluidic ecosystem. P Nat Acad Sci USA 111(9):836–845. https://doi.org/10.1073/pnas.1324044111
Funding
The study was supported by Key Project of Science and Technology of Hunan Branch of China National Tobacco Corporation (16-19Aa02) and the Graduate Student Research Innovation Project in Central South University (No. 2016zzts105).
Author information
Authors and Affiliations
Contributions
H. Y. and J. L. conceived of the experiment. Y. X. and H. Y. performed the experiment. Y. X. analyzed the data and wrote the manuscript. X. L., D. M., J. T., and Y. G. participated in the discussions.
Corresponding authors
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interests.
Electronic supplementary material
ESM 1
(PDF 2523 kb)
Rights and permissions
About this article
Cite this article
Xiao, Y., Liu, X., Meng, D. et al. The role of soil bacterial community during winter fallow period in the incidence of tobacco bacterial wilt disease. Appl Microbiol Biotechnol 102, 2399–2412 (2018). https://doi.org/10.1007/s00253-018-8757-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8757-3