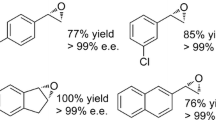
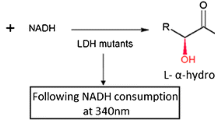
Abstract
Enantiopure styrene oxide (SO) and its derivatives are important building blocks for chiral synthesis. In this study, we developed an attractive “1-pot, 2-step” chemoenzymatic approach for producing enantiopure SO with 100 % theoretical yield. This approach involved asymmetric reduction of α-chloroacetophenone by an alcohol dehydrogenase (ADH; step 1), followed by base-induced ring closure (epoxidation) of enantiopure 2-chloro-1-phenylethanol produced by the ADH (step 2). By-product formation during epoxidation was suppressed to <1 % by adding methyl tert-butyl ether (MTBE) as the second phase. Therefore, with this optimized approach, ADH from Lactobacillus kefir (LkDH) successfully produced 1 M (S)-SO, with 99 % analytical yield and 97.8 % enantiomeric excess (ee). In the preparation of (R)-SO, a semi-rational strategy of active pocket iterative saturation mutagenesis (ISM) was successfully used to inverse the enantioselectivity of LkDH (muDH2, F147L/Y190P/A202F/M206H/V196L/S96D/K97V), which produced the opposite enantiomer (R)-2-chloro-1-phenylethanol. Through the optimized chemoenzymatic approach, muDH2 was successfully used to prepare 1 M (R)-SO, with 98.1 % ee and 99.0 % analytical yield. Our results indicated that this optimized chemoenzymatic approach could be used to produce both enantiomers of SO at concentrations as high as 120 g/L within 14 h, which is the highest concentration as far as we know. MuDH2 obtained through ISM also showed reversed enantioselectivity toward another 13 aromatic ketones, compared with wild-type (WT) LkDH. Furthermore, a molecular docking experiment demonstrated that muDH2 inverted the binding orientation of the substrate, which may be the reason for its inverse enantioselectivity.





Similar content being viewed by others
References
Abokitse K, Hummel W (2003) Cloning, sequence analysis, and heterologous expression of the gene encoding a (S)-specific alcohol dehydrogenase from Rhodococcus erythropolis DSM 43297. Appl Microbiol Biotechnol 62(4):380–386. doi:10.1007/s00253-003-1310-y
Agarwal PK, Webb SP, Hammes-Schiffer S (2000) Computational studies of the mechanism for proton and hydride transfer in liver alcohol dehydrogenase. J Am Chem Soc 122(19):4803–4812. doi:10.1021/ja994456w
Berkessel A, Rollmann C, Chamouleau F, Labs S, May O, Gröger H (2007) Practical two-step synthesis of an enantiopure aliphatic terminal (S)-epoxide based on reduction of haloalkanones with “designer cells”. Adv Synth Catal 349(17-18):2697–2704. doi:10.1002/adsc.200700244
Bisogno FR, Cuetos A, Orden AA, Kurina-Sanz M, Lavandera I, Gotor V (2010) Chemo- and stereodivergent preparation of terminal epoxides and bromohydrins through one-pot biocatalysed reactions: access to enantiopure five- and six-membered N-heterocycles. Adv Synth Catal 352(10):1657–1661. doi:10.1002/adsc.201000353
Bogin O, Peretz M, Burstein Y (1997) Thermoanaerobacter brockii alcohol dehydrogenase: characterization of the active site metal and its ligand amino acids. Protein Sci 6(2):450–458. doi:10.1002/pro.5560060223
Carlsson AJ, Bauer P, Ma H, Widersten M (2012) Obtaining optical purity for product diols in enzyme-catalyzed epoxide hydrolysis: contributions from changes in both enantio- and regioselectivity. Biochemistry 51(38):7627–7637. doi:10.1021/bi3007725
Deng J, Yao Z, Chen K, Yuan YA, Lin J, Wei D (2016) Towards the computational design and engineering of enzyme enantioselectivity: a case study by a carbonyl reductase from Gluconobacter oxydans. J Biotechnol 217:31–40. doi:10.1016/j.jbiotec.2015.11.003
Hamada T, Torii T, Izawa K, Noyori R, Ikariya T (2002) Practical synthesis of optically active styrene oxides via reductive transformation of 2-chloroacetophenones with chiral rhodium catalysts. Org Lett 4(24):4373–4376. doi:10.1021/ol020213o
Hollmann F, Arends IWCE, Holtmann D (2011) Enzymatic reductions for the chemist. Green Chem 13(9):2285. doi:10.1039/c1gc15424a
Jia X, Wang Z, Li Z (2008) Preparation of (S)-2-, 3-, and 4-chlorostyrene oxides with the epoxide hydrolase from Sphingomonas sp. HXN-200. Tetrahedron-Asymmetry 19(4):407–415. doi:10.1016/j.tetasy.2007.12.019
Koska J, Spassov VZ, Maynard AJ, Yan L, Austin N, Flook PK, Venkatachalam CM (2008) Fully automated molecular mechanics based induced fit protein − ligand docking method. J Chem Inf Model 48(10):1965–1973. doi:10.1021/ci800081s
Kotik M, Archelas A, Wohlgemuth R (2012) Epoxide hydrolases and their application in organic synthesis. Curr Org Chem 16(4):451–482. doi:10.2174/138527212799499840
Kuhn D, Kholiq MA, Heinzle E, Bühler B, Schmid A (2010) Intensification and economic and ecological assessment of a biocatalytic oxyfunctionalization process. Green Chem 12(5):815. doi:10.1039/b921896c
Liang J, Jenne S, Mundorff E, Ching C, Gruber J, Krebber A, Huisman G (2009) Ketoreductase polypeptides for the reduction of acetophenones. WO2009036404
Matsumoto K, Oguma T, Katsuki T (2009) Highly enantioselective epoxidation of styrenes catalyzed by proline-derived C1-symmetric titanium(salan) complexes. Angew Chem Int Ed Engl 48(40):7432–7435. doi:10.1002/anie.200903567
Musa MM, Lott N, Laivenieks M, Watanabe L, Vieille C, Phillips RS (2009) A single point mutation reverses the enantiopreference of Thermoanaerobacter ethanolicus secondary alcohol dehydrogenase. ChemCatChem 1(1):89–93. doi:10.1002/cctc.200900033
Nealon CM, Musa MM, Patel JM, Phillips RS (2015) Controlling substrate specificity and stereospecificity of alcohol dehydrogenases. ACS Catal 5(4):2100–2114. doi:10.1021/cs501457v
Niefind K, Müller J, Riebel B, Hummel W, Schomburg D (2003) The crystal structure of R-specific alcohol dehydrogenase from Lactobacillus brevis suggests the structural basis of its metal dependency. J Mol Biol 327(2):317–328. doi:10.1016/s0022-2836(03)00081-0
Noey EL, Tibrewal N, Jimenez-Oses G, Osuna S, Park J, Bond CM, Cascio D, Liang J, Zhang X, Huisman GW, Tang Y, Houk KN (2015) Origins of stereoselectivity in evolved ketoreductases. Proc Natl Acad Sci U S A 112(51):E7065–7072. doi:10.1073/pnas.1507910112
Pace V, Cabrera AC, Ferrario V, Sinisterra JV, Ebert C, Gardossi L, Braiuca P, Alcantara AR (2011) Structural bases for understanding the stereoselectivity in ketone reductions with ADH from Thermus thermophilus: a quantitative model. J Mol Catal B Enzym 70(1–2):23–31. doi:10.1016/j.molcatb.2011.01.017
Poessl TM, Kosjek B, Ellmer U, Gruber CC, Edegger K, Faber K, Hildebrandt P, Bornscheuer UT, Kroutil W (2005) Non-racemic halohydrins via biocatalytic hydrogen-transfer reduction of halo-ketones and one-pot cascade reaction to enantiopure epoxides. Adv Synth Catal 347(14):1827–1834. doi:10.1002/adsc.200505094
Reetz MT, Carballeira JD (2007) Iterative saturation mutagenesis (ISM) for rapid directed evolution of functional enzymes. Nat Protoc 2(4):891–903. doi:10.1038/nprot.2007.72
Talsi EP, Samsonenko DG, Bryliakov KP (2014) Titanium salan catalysts for the asymmetric epoxidation of alkenes: steric and electronic factors governing the activity and enantioselectivity. Chemistry 20(44):14329–14335. doi:10.1002/chem.201404157
Toda H, Ohuchi T, Imae R, Itoh N (2015) Microbial production of aliphatic (S)-epoxyalkanes using styrene monooxygenase of Rhodococcus sp. ST-10 expressed in organic solvent-tolerant Kocuria rhizophila DC2201. Appl Environ Microbiol. doi:10.1128/aem.03405-14
Tokunaga M, Larrow JF, Kakiuchi F, Jacobsen EN (1997) Asymmetric catalysis with water: efficient kinetic resolution of terminal epoxides by means of catalytic hydrolysis. Science 277(5328):936–938. doi:10.1126/science.277.5328.936
Weckbecker A, Hummel W (2006) Cloning, expression, and characterization of an (R)-specific alcohol dehydrogenase from Lactobacillus kefir. Biocatal Biotransform 24(5):380–389. doi:10.1080/10242420600893827
Wu S, Li A, Chin YS, Li Z (2013) Enantioselective hydrolysis of racemic and meso-epoxides with recombinant Escherichia coli expressing epoxide hydrolase from Sphingomonas sp. HXN-200: preparation of epoxides and vicinal diols in higheeand high concentration. ACS Catal 3(4):752–759 doi:10.1021/cs300804v
Wu K, Wang H, Sun H, Wei D (2015) Efficient kinetic resolution of phenyl glycidyl ether by a novel epoxide hydrolase from Tsukamurella paurometabola. Appl Microbiol Biotechnol. doi:10.1007/s00253-015-6716-9, 1–11
Wu K, Chen L, Fan H, Zhao Z, Wang H, Wei D (2016) Synthesis of enantiopure epoxide by ‘one pot’ chemoenzymatic approach using a highly enantioselective dehydrogenase. Tetrahedron Lett 57(8):899–904. doi:10.1016/j.tetlet.2016.01.048
Yoo SS, Park S, Lee EY (2008) Enantioselective resolution of racemic styrene oxide at high concentration using recombinant Pichia pastoris expressing epoxide hydrolase of Rhodotorula glutinis in the presence of surfactant and glycerol. Biotechnol Lett 30(10):1807–1810. doi:10.1007/s10529-008-9762-x
Zhang ZG, Lonsdale R, Sanchis J, Reetz MT (2014) Extreme synergistic mutational effects in the directed evolution of a baeyer-villiger monooxygenase as catalyst for asymmetric sulfoxidation. J Am Chem Soc 136(49):17262–17272. doi:10.1021/ja5098034
Zhu D, Malik HT, Hua L (2006a) Asymmetric ketone reduction by a hyperthermophilic alcohol dehydrogenase. The substrate specificity, enantioselectivity and tolerance of organic solvents. Tetrahedron Asymmetry 17(21):3010–3014. doi:10.1016/j.tetasy.2006.10.042
Zhu D, Yang Y, Buynak JD, Hua L (2006b) Stereoselective ketone reduction by a carbonyl reductase from Sporobolomyces salmonicolor. Substrate specificity, enantioselectivity and enzyme-substrate docking studies. Org Biomol Chem 4(14):2690–2695. doi:10.1039/b606001c
Zhu D, Yang Y, Hua L (2006c) Stereoselective enzymatic synthesis of chiral alcohols with the use of a carbonyl reductase from Candida magnoliae with anti-prelog enantioselectivity. J Org Chem 71(11):4202–4205. doi:10.1021/jo0603328
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21406068/B060804), the Fundamental Research Funds for the Central Universities, and the National Basic Research Program of China (No. 2012CB721103).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Kai Wu and Hualei Wang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 504 kb)
Rights and permissions
About this article
Cite this article
Wu, K., Wang, H., Chen, L. et al. Practical two-step synthesis of enantiopure styrene oxide through an optimized chemoenzymatic approach. Appl Microbiol Biotechnol 100, 8757–8767 (2016). https://doi.org/10.1007/s00253-016-7631-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7631-4