Abstract
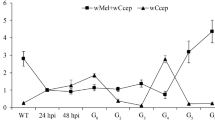
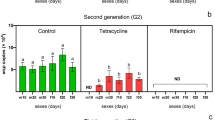
Endosymbiotic bacterium Wolbachia interacts with host in either a mutualistic or parasitic manner. Wolbachia is frequently identified in various arthropod species, and to date, Wolbachia infections have been detected in different insects. Here, we found a triple Wolbachia infection in Homona magnanima, a serious tea pest, and investigated the effects of three infecting Wolbachia strains (wHm-a, -b, and -c) on the host. Starting with the triple-infected host line (Wabc), which was collected in western Tokyo in 1999 and maintained in laboratory, we established an uninfected line (W−) and three singly infected lines (Wa, Wb, and Wc) using antibiotics. Mating experiments with the host lines revealed that only wHm-b induced cytoplasmic incompatibility (CI) in H. magnanima, with the intensities of CI different between the Wb and Wabc lines. Regarding mutualistic effects, wHm-c shortened larval development time and increased pupal weight in both the Wc and Wabc lines to the same extent, whereas no distinct phenotype was observed in lines singly infected with wHm-a. Based on quantitative PCR analysis, Wolbachia density in the Wa line was higher than in the other host lines (p < 0.01, n = 10). Wolbachia density in the Wb line was also higher than in the Wc and Wabc lines, while no difference was observed between the Wc and Wabc lines. These results indicate that the difference in the CI intensity between a single or multiple infection may be attributed to the difference in wHm-b density. However, no correlation was observed between mutualistic effects and Wolbachia density.




Similar content being viewed by others
References
Buchner P (1965) Endosymbiosis of animals with plant microorganisms. John Wiley & Sons Inc., New York
Bourtzis K, Miller TA (2003) Insect symbiosis. CRC Press, Florida
Kikuchi Y, Hosokawa T, Nikoh N, Meng XY, Kamagata Y, Fukatsu T (2009) Host-symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol. 7:2
Roberts LW, Rapmund G, Cadigan Jr FC (1977) Sex ratios in Rickettsia tsutsugamushi-infected and noninfected colonies of Leptotrombidium (Acari: Trombiculidae). J. Med. Entomol. 14:89–92
Gherna RL, Werren JH, Weisburg W, Cote R, Woese CR, Mandelco L, Brenner DJ (1991) Arsenophonus nasoniae gen. nov., sp. nov., the causative agent of the son-killer trait in the parasitic wasp Nasonia vitripennis. Int. J. Syst. Bacteriol. 41:563–565
Werren JH (1997) Biology of Wolbachia. Annu. Rev. Entomol. 42:587–609
Williamson DL, Sakaguchi B, Hackett KJ, Whitcomb RF, Tully JG, Carle P, Bové JM, Adams JR, Konai M, Henegar RB (1999) Spiroplasma poulsonii sp. nov., a new species associated with male-lethality in Drosophila willistoni, a neotropical species of fruit fly. Int. J. Syst. Bacteriol. 49:611–618
Zchori-Fein E, Perlman SJ (2004) Distribution of the bacterial symbiont Cardinium in arthropods. Mol. Ecol. 13:2009–2016
Andreadis TG, Hall DW (1979) Development, ultrastructure, and mode of transmission of Amblyospora sp. (Microspora) in the mosquito. J. Protozool. 26:444–452
Nakanishi K, Hoshino M, Nakai M, Kunimi Y (2008) Novel RNA sequences associated with late male killing in Homona magnanima. Proc. R. Soc. B 275:1249–1254
Stouthamer R, Breeuwer JA, Hurst GD (1999) Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 53:71–102
Zug R, Hammerstein P (2012) Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One 7:e38544
Vavre F, Girin C, Boulétreau M (1999) Phylogenetic status of a fecundity-enhancing Wolbachia that does not induce thelytoky in Trichogramma. Insect Mol. Biol. 8:67–72
Fry AJ, Rand DM (2002) Wolbachia interactions that determine Drosophila melanogaster survival. Evolution 56:1976–1981
Brownlie JC, Cass BN, Riegler M, Witsenburg JJ, Iturbe-Ormaetxe I, McGraw EA, O’Neill SL (2009) Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog. 5:e1000368
Teixeira L, Ferreira A, Ashburner M (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6:e1000002
Bordenstein SR, Werren JH (2000) Do Wolbachia influence fecundity in Nasonia vitripennis? Heredity 84:54–62
Harcombe W, Hoffmann AA (2004) Wolbachia effects in Drosophila melanogaster: in search of fitness benefits. J. Invertebr. Pathol. 87:45–50
Jaenike J, Unckless R, Cockburn SN, Boelio LM, Periman SJ (2010) Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329:212–215
Zhao DX, Zhang XF, Hong XY (2013) Host-symbiont interactions in spider mite Tetranychus truncates doubly infected with Wolbachia and Cardinium. Environ. Entomol. 42:445–452
Kondo N, Ijichi N, Shimada M, Fukatsu T (2002) Prevailing triple infection with Wolbachia in Callosobruchus chinensis (Coleoptera: Bruchidae). Mol. Ecol. 11:167–180
Malloch G, Fenton B, Butcher RD (2000) Molecular evidence for multiple infections of a new subgroup of Wolbachia in the European raspberry beetle Byturus tomentosus. Mol. Ecol. 9:77–90
Reuter M, Keller L (2003) High levels of multiple Wolbachia infection and recombination in the ant Formica exsecta. Mol. Biol. Evol. 20:748–753
Narita S, Nomura M, Kageyama D (2007) Naturally occurring single and double infection with Wolbachia strains in the butterfly Eurema hecabe: transmission efficiencies and population density dynamics of each Wolbachia strain. FEMS Microbiol. Ecol. 61:235–245
Atyame CM, Pasteur N, Dumas E, Tortosa P, Tantely ML, Pocquet N, Licciardi S, Bheecarry A, Zumbo B, Weill M, Duron O (2011) Cytoplasmic incompatibility as a means of controlling Culex pipiens quinquefasciatus mosquito in the islands of the south-western Indian Ocean. PLoS Negl. Trop. Dis. 5:e1440
White JA, Kelly SE, Cockburn SN, Perlman SJ, Hunter MS (2011) Endosymbiont costs and benefits in a parasitoid infected with both Wolbachia and Cardinium. Heredity 106:585–591
Watanabe M, Miura K, Hunter MS, Wajnberg E (2011) Superinfection of cytoplasmic incompatibility-inducing Wolbachia is not additive in Orius strigicollis (Hemiptera: Anthocoridae). Heredity 106:642–648
Kondo N, Shimada M, Fukatsu T (2005) Infection density of Wolbachia endosymbiont affected by co-infection and host genotype. Biol. Lett. 1:488–491
Goto S, Anbutsu H, Fukatsu T (2006) Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Appl. Environ. Microbiol. 72:4805–4810
Morimoto S, Nakai M, Ono A, Kunimi Y (2001) Late male-killing phenomenon found in a Japanese population of the oriental tea tortrix, Homona magnanima (Lepidoptera: Tortricidae). Heredity 87:435–440
Tsugeno Y, Koyama H, Takamatsu T, Nakai M, Kunimi Y, Inoue MN (2017) Identification of an early male-killing agent in the oriental tea tortrix, Homona magnanima. J. Hered. 108:553–560
Dobson SL, Bourtzis K, Braig HR, Jones BF, Zhou W, Rousset F, O'Neill SL (1999) Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 29:153–160
Lalzar I, Friedmann Y, Gottlieb Y (2014) Tissue tropism and vertical transmission of Coxiella in Rhipicephalus sanguineus and Rhipicephalus turanicus ticks. Environ. Microbiol. 16:3657–3668
Zhou W, Rousset F, O’Neill S (1998) Phylogeny and PCR–based classification of Wolbachia strains using wsp gene sequences. Proc. Biol. Sci. 265:509–515
Hurst GDD, Jiggins FM, von der Schulenburg JHG, Bertrand D, West SA, Goriacheva II, Zakharov IA, Werren JH, Stouthamer R, Majerus MEN (1999) Male-killing Wolbachia in two species of insect. Proc. Biol. Sci. 266:735–740
Regnery RL, Spruill CL, Plikaytis BD (1991) Genotypic identification of Rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 173:1576–1589
Noda H, Koizumi Y, Zhang Q, Deng K (2001) Infection density of Wolbachia and incompatibility level in two planthopper species, Laodelphax striatellus and Sogatella furcifera. Insect Biochem. Mol. Biol. 31:727–737
Sanada-Morimura S, Matsumura M, Noda H (2013) Male killing caused by a Spiroplasma symbiont in the small brown planthopper, Laodelphax striatellus. J. Hered. 104:821–829
Noda H, Watanabe K, Kawai S, Yukuhiro F, Miyoshi T, Tomizawa M, Koizumi Y, Nikoh N, Fukatsu T (2012) Bacteriome-associated endosymbionts of the green rice leafhopper Nephotettix cincticeps (Hemiptera: Cicadellidae). Appl. Entomol. Zool. 47:217–225
Baldo L, Hotopp JCD, Jolley KA, Bordenstein SR, Biber SA, Choudhury RR, Hayashi C, Maiden MCJ, Tettelin H, Werren JH (2006) Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 72:7098–7110
Iwata K, Haas-Stapleton E, Kunimi Y, Inoue MN, Nakai M (2017) Midgut-based resistance to oral infection by a nucleopolyhedrovirus in the laboratory-selected strain of the smaller tea tortrix, Adoxophyes honmai (Lepidoptera: Tortricidae). J. Gen. Virol. 98:296–304
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30:2725–2729
Turelli M, Hoffmann AA (1991) Rapid spread of an inherited incompatibility factor in California Drosophila. Nature 353:440–442
Cheng HH (1972) Oviposition and longevity of the dark-sided cutworm, Euxoa messoria (Lepidoptera: Noctuidae), in the laboratory. Can. Entomol. 104:919–925
Hough JA, Pimentel D (1978) Influence of host foliage on development, survival, and fecundity of the gypsy moth. Environ. Entomol. 7:97–102
Honěk A (1993) Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos 66:483–492
Sato T, Oho N, Kodomari S (1980) A granulosis virus of the tea tortrix, Homona magnanima Diaknoff (Lepidoptera: Tortricidae): its pathogenicity and mass-production method. Appl. Entomol. Zool. 15:409–415
Mao HX, Kunimi Y (1991) Pupal mortality of the oriental tea tortrix, Homona magnanima Diakonoff (Lepidoptera: Tortricidae), caused by parasitoids and pathogens. Jpn. J. Appl. Entomol. Zool. 35:241–245
Takatsuka J, Okuno S, Ishii T, Nakai M, Kunimi Y (2010) Fitness-related traits of entomopoxviruses isolated from Adoxophyes honmai (Lepidoptera: Tortricidae) at three localities in Japan. J. Invertebr. Pathol. 105:121–131
Veneti Z, Clark ME, Karr TL, Savakis C, Bourtzis K (2004) Heads or tails: host-parasite interactions in the Drosophila-Wolbachia system. Appl. Environ. Microbiol. 70:5366–5372
Hoffmann AA, Clancy D, Duncan J (1996) Naturally-occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity 76:1–8
McGraw EA, Merritt DJ, Droller JN, O’Neill SL (2002) Wolbachia density and virulence attenuation after transfer into a novel host. Proc. Natl. Acad. Sci. U. S. A. 99:2918–2923
Reynolds KT, Thomson LJ, Hoffmann AA (2003) The effects of host age, host nuclear background and temperature on phenotypic effects of the virulent Wolbachia strain popcorn in Drosophila melanogaster. Genetics 164:1027–1034
Unckless RL, Boelio LM, Herren JK, Jaenike J (2009) Wolbachia as populations within individual insects: causes and consequences of density variation in natural populations. Proc. R. Soc. B 276:2805–2811
Hurst GD, Jiggins FM (2000) Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerg. Infect. Dis. 6:329–336
Sasaki T, Ishikawa H (2000) Transinfection of Wolbachia in the Mediterranean flour moth, Ephestia kuehniella, by embryonic microinjection. Heredity 85:130–135
Sasaki T, Massaki N, Kubo T (2005) Wolbachia variant that induces two distinct reproductive phenotypes in different hosts. Heredity 95:389–393
Author information
Authors and Affiliations
Corresponding author
Additional information
Arai and Hirano equally contribute to this paper
Rights and permissions
About this article
Cite this article
Arai, H., Hirano, T., Akizuki, N. et al. Multiple Infection and Reproductive Manipulations of Wolbachia in Homona magnanima (Lepidoptera: Tortricidae). Microb Ecol 77, 257–266 (2019). https://doi.org/10.1007/s00248-018-1210-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-018-1210-4