Abstract
Background
Although acute neurologic impairment might be transient, other long-term effects can be observed with mild traumatic brain injury. However, when pediatric patients with mild traumatic brain injury present for medical care, conventional imaging with CT and MR imaging often does not reveal abnormalities.
Objective
To determine whether edge density imaging can separate pediatric mild traumatic brain injury from typically developing controls.
Materials and methods
Subjects were recruited as part of the “Therapeutic Resources for Attention Improvement using Neuroimaging in Traumatic Brain Injury” (TRAIN-TBI) study. We included 24 adolescents (χ=14.1 years of age, σ=1.6 years, range 10–16 years), 14 with mild traumatic brain injury (TBI) and 10 typically developing controls. Neurocognitive assessments included the pediatric version of the California Verbal Learning Test (CVLT) and the Attention Network Task (ANT). Diffusion MR imaging was acquired on a 3-tesla (T) scanner. Edge density images were computed utilizing fiber tractography. Principal component analysis (PCA) and support vector machines (SVM) were used in an exploratory analysis to separate mild TBI and control groups. The diagnostic accuracy of edge density imaging, neurocognitive tests, and fractional anisotropy (FA) from diffusion tensor imaging (DTI) was computed with two-sample t-tests and receiver operating characteristic (ROC) metrics.
Results
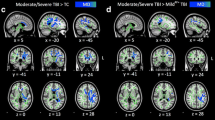
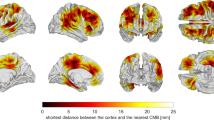
Support vector machine–principal component analysis of edge density imaging maps identified three white matter regions distinguishing pediatric mild TBI from controls. The bilateral tapetum, sagittal stratum, and callosal splenium identified mild TBI subjects with sensitivity of 79% and specificity of 100%. Accuracy from the area under the ROC curve (AUC) was 94%. Neurocognitive testing provided an AUC of 61% (CVLT) and 71% (ANT). Fractional anisotropy yielded an AUC of 48%.
Conclusion
In this proof-of-concept study, we show that edge density imaging is a new form of connectome mapping that provides better diagnostic delineation between pediatric mild TBI and healthy controls than DTI or neurocognitive assessments of memory or attention.



Similar content being viewed by others
References
Dewan MC, Mummareddy N, Wellons JC, Bonfield CM (2016) Epidemiology of global pediatric traumatic brain injury: qualitative review. World Neurosurg 91:497–509.e1
Alexiou G, Prodromou N, Sfakianos G (2011) Pediatric head trauma. J Emerg Trauma Shock 4:403
Pfister T, Pfister K, Hagel B et al (2016) The incidence of concussion in youth sports: a systematic review and meta-analysis. Br J Sports Med 50:292–297
Kay T, Harrington DE, Adams R et al (1993) Definition of mild traumatic brain injury. J Head Trauma Rehabil 8:86–87
Guo X, Edmed SL, Anderson V, Kenardy J (2017) Neurocognitive predictors of posttraumatic stress disorder symptoms in children 6 months after traumatic brain injury: a prospective study. Neuropsychology 31:84–92
Tkachenko N, Singh K, Hasanaj L et al (2016) Sleep disorders associated with mild traumatic brain injury using sport concussion assessment tool 3. Pediatr Neurol 57:46–50.e1
Ellis MJ, Ritchie LJ, Koltek M et al (2015) Psychiatric outcomes after pediatric sports-related concussion. J Neurosurg Pediatr 16:709–718
Buttram SDW, Garcia-Filion P, Miller J et al (2015) Computed tomography vs. magnetic resonance imaging for identifying acute lesions in pediatric traumatic brain injury. Hosp Pediatr 5:79–84
Eierud C, Craddock RC, Fletcher S et al (2014) Neuroimaging after mild traumatic brain injury: review and meta-analysis. Neuroimage Clin 4:283–294
Smits M, Houston GC, Dippel DWJ et al (2011) Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiology 53:553–563
Babcock L, Yuan W, Leach J et al (2015) White matter alterations in youth with acute mild traumatic brain injury. J Pediatr Rehabil Med 8:285–296
Roberts RM, Mathias JL, Rose SE (2014) Diffusion tensor imaging (DTI) findings following pediatric non-penetrating TBI: a meta-analysis. Dev Neuropsychol 39:600–637
Sporns O, Tononi G, Kötter R (2005) The human connectome: a structural description of the human brain. PLoS Comput Biol 1:e42
Jbabdi S, Behrens TEJ, Smith SM (2010) Crossing fibres in tract-based spatial statistics. Neuroimage 49:249–256
Calamante F, Tournier J-D, Jackson GD, Connelly A (2010) Track-density imaging (TDI): super-resolution white matter imaging using whole-brain track-density mapping. Neuroimage 53:1233–1243
Calamante F, Tournier J-D, Heidemann RM et al (2011) Track density imaging (TDI): validation of super resolution property. Neuroimage 56:1259–1266
Payabvash S, Palacios EM, Owen JP et al (2019) White matter connectome edge density in children with autism spectrum disorders: potential imaging biomarkers using machine-learning models. Brain Connect 9:209–220
Owen JP, Wang MB, Mukherjee P (2016) Periventricular white matter is a nexus for network connectivity in the human brain. Brain Connect 6:548–557
Owen JP, Chang YS, Mukherjee P (2015) Edge density imaging: mapping the anatomic embedding of the structural connectome within the white matter of the human brain. Neuroimage 109:402–417
King NS, Crawford S, Wenden FJ et al (1995) The Rivermead post concussion symptoms questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol 242:587–592
Beers SR, Wisniewski SR, Garcia-Filion P et al (2012) Validity of a pediatric version of the Glasgow outcome scale–extended. J Neurotrauma 29:1126–1139
Baker DA, Connery AK, Kirk JW, Kirkwood MW (2014) Embedded performance validity indicators within the California verbal learning test, children’s version. Clin Neuropsychol 28:116–127
Fan J, McCandliss BD, Sommer T et al (2002) Testing the efficiency and independence of attentional networks. J Cogn Neurosci 14:340–347
Haacke EM, Duhaime AC, Gean AD et al (2010) Common data elements in radiologic imaging of traumatic brain injury. J Magn Reson Imaging 32:516–543
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841
Fischl B, Salat DH, van der Kouwe AJW et al (2004) Sequence-independent segmentation of magnetic resonance images. Neuroimage 23:S69–S84
Desikan RS, Segonne F, Fischl B et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980
Behrens TE, Berg HJ, Jbabdi S et al (2007) Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 34:144–155
Wakana S, Jiang H, Nagae-Poetscher LM et al (2004) Fiber tract-based atlas of human white matter anatomy. Radiology 230:77–87
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. R Stat Soc 57:289–300
Toga AW, Thompson PM (2001) The role of image registration in brain mapping. Image Vis Comput 19:3–24
Georgieva P, De la Torre F (2013) Robust principal component analysis for brain imaging. In: Mladenov V, Koprinkova-Hristova P, Palm G et al (eds) Artificial neural networks and machine learning — ICANN 2013. Springer, Berlin, pp 288–295
Abdullah N, Ngah UK, Aziz SA (2011) Image classification of brain MRI using support vector machine. IEEE, pp 242–247
Zou KH, O’Malley AJ, Mauri L (2007) Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation 115:654–657
Habeck C, Stern Y, Alzheimer’s Disease Neuroimaging Initiative (2010) Multivariate data analysis for neuroimaging data: overview and application to Alzheimer’s disease. Cell Biochem Biophys 58:53–67
Jenkinson M, Beckmann CF, Behrens TEJ et al (2012) FSL. Neuroimage 62:782–790
Holmes CJ, Hoge R, Collins L et al (1998) Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 22:324–333
Levin HS, Wilde EA, Hanten G et al (2011) Mental state attributions and diffusion tensor imaging after traumatic brain injury in children. Dev Neuropsychol 36:273–287
Ewing-Cobbs L, Prasad MR, Swank P et al (2008) Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. Neuroimage 42:1305–1315
Wozniak J, Krach L, Ward E et al (2007) Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch Clin Neuropsychol 22:555–568
Fakhran S, Yaeger K, Collins M, Alhilali L (2014) Sex differences in white matter abnormalities after mild traumatic brain injury: localization and correlation with outcome. Radiology 272:815–823
McCauley SR, Wilde EA, Bigler ED et al (2011) Diffusion tensor imaging of incentive effects in prospective memory after pediatric traumatic brain injury. J Neurotrauma 28:503–516
Oni MB, Wilde EA, Bigler ED et al (2010) Diffusion tensor imaging analysis of frontal lobes in pediatric traumatic brain injury. J Child Neurol 25:976–984
Wilde EA, Bigler ED, Haider JM et al (2006) Vulnerability of the anterior commissure in moderate to severe pediatric traumatic brain injury. J Child Neurol 21:769–776
Cicuendez M, Castaño-León A, Ramos A et al (2017) Prognostic value of corpus callosum injuries in severe head trauma. Acta Neurochir 159:25–32
Jellison BJ, Field AS, Medow J et al (2004) Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol 25:356–369
Mayer AR, Ling JM, Yang Z et al (2012) Diffusion abnormalities in pediatric mild traumatic brain injury. J Neurosci 32:17961–17969
DeMaster D, Johnson C, Juranek J, Ewing-Cobbs L (2017) Memory and the hippocampal formation following pediatric traumatic brain injury. Brain Behav 7:e00832
Wu TC, Wilde EA, Bigler ED et al (2010) Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Dev Neurosci 32:361–373
Mioni G, Grondin S, Stablum F (2014) Temporal dysfunction in traumatic brain injury patients: primary or secondary impairment? Front Hum Neurosci 8:269
Buethe J, Nazarian J, Kalisz K, Wintermark M (2016) Neuroimaging wisely. AJNR Am J Neuroradiol 37:2182–2188
Wintermark M, Sanelli PC, Anzai Y et al (2015) Imaging evidence and recommendations for traumatic brain injury: advanced neuro- and neurovascular imaging techniques. AJNR Am J Neuroradiol 36:E1–E11
Bach M, Laun FB, Leemans A et al (2014) Methodological considerations on tract-based spatial statistics (TBSS). Neuroimage 100:358–369
Peled S, Yeshurun Y (2001) Superresolution in MRI: application to human white matter fiber tract visualization by diffusion tensor imaging. Magn Reson Med 45:29–35
Acknowledgments
The TRAIN-TBI project was generously supported by a gift from Dennis J. & Shannon Wong. Dr. Raji was supported by a training grant from the National Institute of Biomedical Imaging and Bioengineering (NIH T32 EB001631), administered by the UCSF Department of Radiology and Biomedical Imaging, and the American Society of Neuroradiology Boerger Research Grant. He is currently supported by additional grants from the Radiological Society of North America Research Scholar Award and WUSTL NIH KL2 Grant (KL2 TR000450 – ICTS Multidisciplinary Clinical Research Career Development Program). Dr. Mukherjee received support from the National Institute of Neurological Disorders and Stroke (NIH R01 NS060776).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raji, C.A., Wang, M.B., Nguyen, N. et al. Connectome mapping with edge density imaging differentiates pediatric mild traumatic brain injury from typically developing controls: proof of concept. Pediatr Radiol 50, 1594–1601 (2020). https://doi.org/10.1007/s00247-020-04743-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-020-04743-9