Abstract
Background
Spinal muscular atrophy (SMA) is an autosomal-recessive neuromuscular disorder resulting in progressive muscle weakness. In December 2016, the U.S. Food and Drug Administration approved the first treatment for SMA, a drug named nusinersen (Spinraza) that is administered intrathecally. However many children with SMA have neuromuscular scoliosis or spinal instrumentation resulting in challenging intrathecal access. Therefore alternative routes must be considered in these complex patients.
Objective
To investigate routes of drug access, we reviewed our institutional experience of administering intrathecal nusinersen in all children with spinal muscular atrophy regardless of spinal anatomy or instrumentation.
Materials and methods
We reviewed children with SMA who were referred for intrathecal nusinersen injections from March to December 2017 at our institution. In select children with spinal hardware, spinal imaging was requested to facilitate pre-procedure planning. Standard equipment for intrathecal injections was utilized. All children were followed up by their referring neurologist.
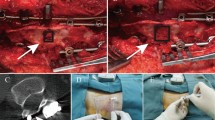
Results
A total of 104 intrathecal nusinersen injections were performed in 26 children with 100% technical success. Sixty procedures were performed without pre-procedural imaging and via standard interspinous technique. The remaining 44 procedures were performed in 11 complex (i.e. neuromuscular scoliosis or spinal instrumentation) patients requiring pre-procedural imaging for planning purposes. Nineteen of the 44 complex procedures were performed via standard interspinous technique from L2 to S1. Twenty-two of the 44 complex procedures were performed using a neural-foraminal approach from L3 to L5. Three of the 44 complex procedures were performed via cervical puncture technique. There were no immediate or long-term complications but there was one child with short-term complications of meningismus and back pain at the injection site.
Conclusion
Although we achieved 100% technical success in intrathecal nusinersen administration, our practices evolved during the course of this study. As a result of our early experience we developed an algorithm to assist in promoting safe and effective nusinersen administration in children with spinal muscular atrophy regardless of SMA type, abnormal spinal anatomy and complex spinal instrumentation.







Similar content being viewed by others
References
Crawford TO, Pardo CA (1996) The neurobiology of childhood spinal muscular atrophy. Neurobiol Dis 3:97–110
Pearn J (1980) Classification of spinal muscular atrophies. Lancet 1:919–922
Prior TW, Snyder PJ, Rink BD et al (2010) Newborn and carrier screening for spinal muscular atrophy. Am J Med Genet 152A:1608–1616
Farooq FT, Holcik M, MacKenzie A (2013) Spinal muscular atrophy: classification, diagnosis, background, molecular mechanism and development of therapeutics. Neurodegener Dis. https://doi.org/10.5772/53800
Prior TW, Finanger E (2000) Spinal muscular atrophy. In: Adam MP, Ardinger HH, Pagon RA et al (eds) GeneReviews. University of Washington, Seattle
Zerres K, Rudnik-Schöneborn S, Forrest E et al (1997) A collaborative study on the natural history of childhood and juvenile onset proximal spinal muscular atrophy (type II and III SMA): 569 patients. J Neurol Sci 146:67–72
U. S. Food and Drug Administration (2018) Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=209531. Accessed 14 June 2018
Chiriboga CA, Swoboda KJ, Darras BT et al (2016) Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy. Neurology 86:890–897
Pechmann A, Langer T, Wider S, Kirschner J (2017) Single-center experience with intrathecal administration of nusinersen in children with spinal muscular atrophy type 1. Eur J Paediatr Neurol 22:122–127
Thacker M, Hui J, Wong H et al (2002) Spinal fusion and instrumentation for paediatric neuromuscular scoliosis: retrospective review. J Orthop Surg 10:144–151
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Mousa, M.A., Aria, D.J., Schaefer, C.M. et al. A comprehensive institutional overview of intrathecal nusinersen injections for spinal muscular atrophy. Pediatr Radiol 48, 1797–1805 (2018). https://doi.org/10.1007/s00247-018-4206-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-018-4206-9