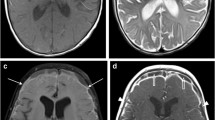
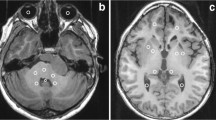
Abstract
Background
Gadoteric acid is a paramagnetic gadolinium macrocyclic contrast agent approved for use in MRI of cerebral and spinal lesions and for body imaging.
Objective
To investigate the safety and efficacy of gadoteric acid in children by extensively reviewing clinical and post-marketing observational studies.
Materials and methods
Data were collected from 3,810 children (ages 3 days to 17 years) investigated in seven clinical trials of central nervous system (CNS) imaging (n = 141) and six post-marketing observational studies of CNS, musculoskeletal and whole-body MR imaging (n = 3,669). Of these, 3,569 children were 2–17 years of age and 241 were younger than 2 years. Gadoteric acid was generally administered at a dose of 0.1 mmol/kg. We evaluated image quality, lesion detection and border delineation, and the safety of gadoteric acid. We also reviewed post-marketing pharmacovigilance experience.
Results
Consistent with findings in adults, gadoteric acid was effective in children for improving image quality compared with T1-W unenhanced sequences, providing diagnostic improvement, and often influencing the therapeutic approach, resulting in treatment modifications. In studies assessing neurological tumors, gadoteric acid improved border delineation, internal morphology and contrast enhancement compared to unenhanced MR imaging. Gadoteric acid has a well-established safety profile. Among all studies, a total of 10 children experienced 20 adverse events, 7 of which were thought to be related to gadoteric acid. No serious adverse events were reported in any study. Post-marketing pharmacovigilance experience did not find any specific safety concern.
Conclusion
Gadoteric acid was associated with improved lesion detection and delineation and is an effective and well-tolerated contrast agent for use in children.
Similar content being viewed by others
References
MacKenzie JD, Vasanawala SS (2010) State-of-the-art in pediatric body and musculoskeletal magnetic resonance imaging. Semin Ultrasound CT MR 31:86–99
Hervé-Somma CM, Sebag GH, Prieur AM et al (1992) Juvenile rheumatoid arthritis of the knee: MR evaluation with Gd-DOTA. Radiology 182:93–98
Bonnerot V, Sebag G, de Montalembert M et al (1994) Gadolinium-DOTA enhanced MRI of painful osseous crises in children with sickle cell anemia. Pediatr Radiol 24:92–95
Ducou le Pointe H, Haddad S, Silberman B et al (1994) Legg–Perthes–Calve disease: staging by MRI using gadolinium. Pediatr Radiol 24:88–91
Hanquinet S, Christophe C, Greef DD et al (1996) Clinical evaluation of gadodiamide injection in paediatric MR imaging. Pediatr Radiol 26:806–810
Lundby B, Gordon P, Hugo F (1996) MRI in children given gadodiamide injection: safety and efficacy in CNS and body indications. Eur J Radiol 23:190–196
Lowe LH, Kearns GL, Wible JH Jr (2006) The safety and efficacy of neuroimaging with gadoversetamide injection in pediatric patients. Curr Med Res Opin 22:2515–2524
Barkovich A, Raybaud C (2011) Pediatric neuroimaging, 5th edn. Lippincott Williams & Wilkins, Philadelphia
Rao P (2008) Role of MRI in paediatric neurooncology. Eur J Radiol 68:259–270
Colosimo C, Demaerel P, Tortori-Donati P et al (2005) Comparison of gadobenate dimeglumine (Gd-BOPTA) with gadopentetate dimeglumine (Gd-DTPA) for enhanced MR imaging of brain and spine tumors in children. Pediatr Radiol 35:501–510
Balassy C, Hörmann M (2008) Role of MRI in paediatric musculoskeletal conditions. Eur J Radiol 68:245–258
Riccabona M (2008) MRI in neonates, infants and children — overuse or still insufficient availability for paediatric needs? Eur J Radiol 68:189–190
Dagia C, Ditchfield M (2008) 3T MRI in paediatrics: challenges and clinical applications. Eur J Radiol 68:309–319
Port M, Idée JM, Medina C et al (2008) Efficiency thermodynamics and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review. Biometals 21:469–490
Fretellier N, Idée JM, Bruneval P et al (2012) Hyperphosphataemia sensitizes renally impaired rats to the profibrotic effects of gadodiamide. Brit J Pharmacol 165:1151–1162
Haylor J, Schroeder J, Wagner B et al (2012) Skin gadolinium following use of MR contrast agents in a rat model of nephrogenic systemic fibrosis. Radiology 263:107–116
Grobner T (2006) Gadolinium — a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 21:1104–1108
Kang JW, Lim TH, Choi CG et al (2010) Evaluation of contrast-enhanced magnetic resonance angiography (MRA) using Gd-DOTA compared with time-of-flight MRA in the diagnosis of clinically significant non-coronary arterial disease. Eur Radiol 20:1934–1944
Maurer M, Heine O, Wolf M et al (2012) Tolerability and diagnostic value of gadoteric acid in the general population and in patients with risk factors: results in more than 84,000 patients. Eur J Radiol 81:885–890
Ishiguchi T, Takahashi S (2010) Safety of gadoterate meglumine (Gd-DOTA) as a contrast agent for magnetic resonance imaging: results of a post-marketing surveillance study in Japan. Drugs R D 10:133–145
Meng H, Grosse-Wortmann L (2012) Gadolinium in pediatric cardiovascular magnetic resonance: what we know and how we practice. J Cardiovasc Magn Reson 14:56
Neiss AC, Le Mignon MM, Vitry A et al (1991) Efficacy and safety of DOTA-Gd from a European multicenter survey. Preliminary results on 4,169 cases. Rev Im Med 3:383–387
Briand Y, Neiss AC, Vitry A et al (1992) Efficacy and safety of the macrocyclic complex Gd-DOTA in children: results of a multi-centre study. Proceedings of the 29th Congress of the European Society of Pediatric Radiology, 128
Pracros JP, de la Garanderie J (2012) The SECURE study: observational post-marketing study on the safety of meglumine gadoterate — interim analysis of 972 children. Pediatr Radiol 42:S511–S512
Emond S, Brunelle F (2011) Gd-DOTA administration at MRI in children younger than 18 months of age: immediate adverse reactions. Pediatr Radiol 41:1401–1406
Pathkar D (2012) SECURE study (abstract): observational post-marketing study on the safety of meglumine gadoterate (Gd-DOTA) — interim analysis on 21,447 patients. J Med Imaging Radiat Oncol 56:134
Pearce MS, Salotti JA, Little MP et al (2012) Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380:499–505
Ylänen K, Poutanen T, Savikurki-Heikkilä P et al (2013) Cardiac magnetic resonance imaging in the evaluation of the late effects of anthracyclines among long-term survivors of childhood cancer. J Am Coll Cardiol 61:1539–1547
Martí-Bonmatí L, Vega T, Benito C et al (2000) Safety and efficacy of Omniscan (gadodiamide injection) at 0.1 mmol/kg for MRI in infants younger than 6 months of age: phase III open multicenter study. Invest Radiol 35:141–147
Sebag G, Ducou Le Pointe H, Klein I et al (1997) Dynamic gadolinium-enhanced subtraction MR imaging — a simple technique for the early diagnosis of Legg–Calvé–Perthes disease: preliminary results. Pediatr Radiol 27:216–220
Dillman JR, Hernandez RJ (2009) MRI of Legg–Calve–Perthes disease. AJR Am J Roentgenol 193:1394–1407
Hundley WG, Bluemke DA, Finn JP et al (2010) ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance. A report of the American College of Cardiology foundation task force on expert consensus documents. Circulation 121:2462–2508
Reiter T, Ritter O, Prince MR et al (2012) Minimizing risk of nephrogenic systemic fibrosis in cardiovascular magnetic resonance. J Cardiovasc Magn Reson 14:31
Weller A, Barber JL, Olsen OE (2014) Gadolinium and nephrogenic systemic fibrosis: an update. Pediatr Nephrol 29:1927–1937
Mendichovszky IA, Marks SD, Simcock CM et al (2008) Gadolinium and nephrogenic systemic fibrosis: time to tighten practice. Pediatr Radiol 38:489–496
Nardone B, Saddleton E, Laumann AE et al (2014) Pediatric nephrogenic systemic fibrosis is rarely reported: a RADAR report. Pediatr Radiol 44:173–180
Elmholdt TR, Pedersen M, Jorgensen B et al (2011) Nephrogenic systemic fibrosis is found only among gadolinium-exposed patients with renal insufficiency: a case–control study from Denmark. Br J Dermatol 165:828–836
Elmholdt TR, Olesen ABB, Jorgensen B et al (2013) Nephrogenic systemic fibrosis in Denmark — a nationwide investigation. PLoSOne 8, e82037
Acknowledgments
The authors wish to thank all the participating sites from these clinical trials and post-marketing observational studies supported by the Guerbet Group.
Conflicts of interest
Drs. Balassy, Roberts and Miller were provided data from published and unpublished studies and post-marketing pharmacovigilance studies by Guerbet. Dr. Roberts has served as a paid consultant for Guerbet but did not receive any support related to this specific paper. Dr. Balassy has received honoraria from Guerbet for speaking engagements but did not receive any support related to this specific paper. Dr. Miller has not received financial support from Guerbet.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balassy, C., Roberts, D. & Miller, S.F. Safety and efficacy of gadoteric acid in pediatric magnetic resonance imaging: overview of clinical trials and post-marketing studies. Pediatr Radiol 45, 1831–1841 (2015). https://doi.org/10.1007/s00247-015-3394-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-015-3394-9