Abstract
Background
To study early neurodevelopment in preterm infants, evaluation of brain maturation and injury is increasingly performed using diffusion tensor imaging, for which the reliability of underlying data is paramount.
Objective
To review the literature to evaluate acquisition and processing methodology in diffusion tensor imaging studies of preterm infants.
Materials and methods
We searched the Embase, Medline, Web of Science and Cochrane databases for relevant papers published between 2003 and 2013. The following keywords were included in our search: prematurity, neuroimaging, brain, and diffusion tensor imaging.
Results
We found 74 diffusion tensor imaging studies in preterm infants meeting our inclusion criteria. There was wide variation in acquisition and processing methodology, and we found incomplete reporting of these settings. Nineteen studies (26%) reported the use of neonatal hardware. Data quality assessment was not reported in 13 (18%) studies. Artefacts-correction and data-exclusion was not reported in 33 (45%) and 18 (24%) studies, respectively. Tensor estimation algorithms were reported in 56 (76%) studies but were often suboptimal.
Conclusion
Diffusion tensor imaging acquisition and processing settings are incompletely described in current literature, vary considerably, and frequently do not meet the highest standards.
Similar content being viewed by others
Introduction
The rate of premature birth is rising globally [1]. Although improvements in obstetric and neonatal care have resulted in increased survival rates, neurodevelopmental outcome remains a source of concern because many preterm infants have neuromotor, cognitive and behavioral disabilities that persist in later life [2, 3]. White matter injury is suggested to account for many neurological sequelae among preterm infants, and although cystic periventricular leukomalacia is becoming less common, diffuse non-cystic white matter changes such as alterations in signal intensity and punctate white matter lesions are frequently observed [4–7]. Major changes of fetal white matter take place during the final stages of a normal pregnancy [8]. Infants born preterm undergo these changes in a high-risk extra-uterine environment, which poses risks for normal brain ontogenesis. Diffusion tensor imaging allows us to objectively assess these (microstructural) changes by mapping restricted random motion of water molecules within white matter tissue in vivo [9, 10].
Objective quantification of white matter microstructure and integrity using diffusion tensor imaging (DTI) may elucidate the impact of preterm birth and related sequelae on neurodevelopment, and DTI has the potential to provide early biomarkers of subsequent neurodevelopmental outcome [5, 7, 11–13]. Sophisticated applications of diffusion tensor imaging such as voxel-based analyses and fiber tractography enable visualization and quantification of specific white matter tracts in vivo. Several studies using these techniques have provided important insights into brain development and the impact of injury on functional outcome [14–18]. Recent projects to explore whole-brain connectivity are very promising because mapping neural circuits may help in the understanding of injury mechanisms responsible for neurocognitive impairment [19–21].
However, brain imaging in this specific vulnerable population is quite challenging. Obtaining good-quality data is complicated by the fact that diffusion tensor imaging is intrinsically highly sensitive to artefacts [22–24] and these infants tend to move more and have smaller head sizes and higher heart- and breathing rates than adults [25, 26]. The preterm infant population should be regarded as one of the most challenging patient groups to image using diffusion tensor imaging, and therefore requires maximal awareness of the acquisition and processing steps that determine data quality. Obtaining reliable diffusion tensor imaging data in this specific population can only be achieved when acquisition, quality assessment and data processing steps meet the highest standards possible.
Recently we demonstrated that good-quality diffusion tensor imaging (DTI) data and a well-informed choice of processing methodology have a serious influence on tract characteristics derived from neonatal DTI datasets [27]. Among others, different tensor estimation methods handle outliers and errors differently, and because datasets obtained from preterm infants generally contain a large number of artefacts, this kind of methodological considerations could have a major influence on study results.
The purpose of this study is to evaluate information obtained from diffusion tensor imaging studies of preterm infants, with a focus on acquisition settings, processing methodology and data quality assessment. Therefore, we conducted a systematic review of the literature.
Materials and methods
The Embase, Medline, Web of Science and Cochrane database were systematically searched for relevant papers published between 2003 and September 2013 by two reviewers (K.P., A.P.), each with more than 3 years of experience in neonatal diffusion tensor imaging. The search was performed Oct. 5, 2013, and included synonyms and combinations of the following keywords: prematurity, neuroimaging, brain and diffusion tensor imaging. We included English-written studies in healthy and non-healthy infants. Non-human research, case reports, reviews and editorials were excluded. Studies were considered relevant when they met the following criteria: (1) they included preterm infants born at <32 weeks’ gestation, (2) MRI was performed within the first 28 days after term-equivalent age, and (3) diffusion tensor imaging was incorporated in study design and discussed in the results.
We extracted information regarding:
-
(1)
The use of a neonatal-specific head coil or an MRI-compatible neonatal incubator with a dedicated neonatal head coil, and the use of sedative drugs prior to diffusion tensor imaging acquisition.
-
(2)
Acquisition parameters with regard to diffusion tensor imaging analysis (magnetic field strength, number of gradient directions, b-value, number of non-diffusion-weighted images).
-
(3)
Processing methods (assessment of diffusion tensor imaging data quality, correction for motion and distortions, methods of diffusion tensor estimation and data analysis).
Results
The initial search resulted in 763 articles. All titles and abstracts were screened for relevance, after which the full text versions of 170 seemingly relevant articles were read. Seventy-four articles met our inclusion criteria (Fig. 1). A summary of these is given in Table 1.
Dedicated neonatal MR imaging
Nineteen studies (26%) reported the use of dedicated neonatal scanning equipment; 19 (26%) papers reported the use of a neonatal head coil, which was installed in an MRI-compatible incubator in 18 (24%) of the cases. Sedative drugs were administered prior to scanning in 28 (38%) studies.
Diffusion tensor imaging data acquisition parameters
Seventy-two studies (97%) reported the number of gradient directions at which diffusion tensor imaging (DTI) was performed; this number ranged from 6 to 44, with an average of 18 directions per scan. B values were reported in 71 studies (96%), and most were 600–1,000 s/mm2 (range 350–3,000, average 734 s/mm2, median 700 s/mm2). Number of non-diffusion-weighted images (b = 0) was reported in 57 (77%) studies, mostly limited to one or two b = 0 images per scan (range 1–16, average 1.47).
The static field strength of the MRI scanners was reported in 69 (93%) studies. The most frequently used MRI scanners were 1.5 tesla (n = 44, 60%), followed by 3 tesla (n = 25, 34%).
Processing methods
Sixty-one studies (82%) reported quality assessment of the diffusion-weighted images. Forty-eight studies (65%) reported visual inspection of diffusion data, and three studies (4.1%) reported standardized software-driven quality assessment. Eleven studies (15%) reported quality assessment without further elaboration on how this was performed.
Fifty-six (76%) studies reported exclusion of datasets with insufficient quality. Specific correction methods were applied in 41 studies (55%); this was mostly restricted to correction for motion artefacts (n = 27, 37%) and eddy currents (n = 33, 45%). One study (1.4%) reported the use of automatic detection of outliers (corrupted slices as a result of artefacts or signal-loss) before tensor estimation [74].
Description of the diffusion tensor methodology was available in 56 papers (76%). Among studies that did describe tensor estimation methodology, linear least square and weighted linear least square were most frequently used (n = 29, 39% and n = 23, 31%, respectively).
Region-of-interest analysis was the most frequently used method of analysis (n = 37, 50%). Fiber tractography was applied in 25 studies (34%), of which 13 studies (18%) performed probabilistic tractography, 10 (14%) deterministic tractography and three (4.1%) did not describe which tractography approach was used. Voxel-wise analysis of diffusion data using tracts-based spatial statistics (TBSS) was performed in 15 studies (20.3%).
Discussion
This systematic review demonstrates wide variation among preterm neonatal diffusion tensor imaging studies in hardware setup, acquisition parameters and post-processing settings. Many papers had an incomplete description of these matters.
Acquisition settings
In most studies field strength, b-values and number of directions were reported, and both gradient strength and number of diffusion directions tended to increase over the years. However, reported acquisition parameters differed considerably among the studies. Even when evaluating settings for each year of publication separately, large differences among studies existed in the number of gradient directions and height of b values.
Usage of dedicated neonatal equipment such as specialized neonatal head coils and MRI-compatible incubators was only reported in a minority of studies. MRI-compatible incubators provide a safe and comfortable environment and might therefore reduce subject motion during acquisition. Because our results show that the majority of studies scan without using sedation, a comfortable environment is indispensable to keep the child comfortable and asleep during diffusion tensor imaging acquisition. Furthermore, using smaller head coils, adapted to the characteristics of the preterm brain, might result in higher signal-to-noise ratio [95, 96]. However, because signal-to-noise ratio depends on other features as well, it remains debatable whether dedicated neonatal head coils always provide the best signal-to-noise ratio. For further evaluation of benefits and limitations provided by specific neonatal equipment, it is important that research groups describe which scanning equipment was used and how this impacted scanning convenience and data quality.
Quality assessment of diffusion tensor imaging data
For diffusion tensor imaging, it is known that even optimal equipment and acquisition parameters cannot guarantee appropriate data quality because diffusion tensor imaging is highly sensitive to artefacts. Frequent occurrence of motion during acquisition among preterm infants can result in signal dropout, misalignment of slices, and signal intensity inhomogeneity. In addition, the echo-planar imaging sequence frequently used in neonatal neuroimaging is susceptible to inhomogeneity at air–tissue boundaries [96]. Therefore well-informed processing steps to detect and correct image distortions properly are essential in neonatal diffusion tensor imaging. In a considerable number of studies, information regarding any kind of quality assessment was missing. When quality assessment was stated, detailed description of methodology was frequently not provided. Comprehensive information about precise visual inspection methodology is valuable because different visual inspection strategies might yield different results. Color maps, for example, can be very useful to identify corrupted data but often fail to display signal loss if it is limited to a small number of directions. Careful visual inspection of raw diffusion data in three orthogonal planes by an experienced observer seems to be more effective for this purpose [97]. Further software-based assessment of diffusion tensor imaging quality can reveal additional unobserved image distortions by pointing out more-dispersed signal loss and less-visible artefacts. Our results show that software-driven quality assessment is performed in a limited number of studies. Because there seems to be no consensus regarding assessment of diffusion tensor imaging data quality, a combined approach using multiple methods seems preferable. Such strategies are hardly reported in current neonatal diffusion tensor imaging literature.
When structured quality assessment is extensively performed, it is important to report this. For instance, because of the high likelihood of movement artefacts and signal loss of preterm brain diffusion tensor imaging data, it is often necessary to exclude diffusion data or even complete diffusion tensor imaging scans entirely from analysis to ensure reliability of results. Exclusion of datasets was not reported in 24.3% of the studies included in our review.
Processing methodology
The influence of the chosen tensor estimation methodology on data quality is important to consider because different algorithms address outliers and errors differently [98, 99]. Appropriate algorithms for tensor estimation are crucial in premature infants because reliability of diffusion tensor imaging data depends on how corrupted slices or directions are dealt with. Our literature search showed that information regarding tensor algorithms was not provided in a considerable number of studies and that fast but less accurate tensor algorithms were most frequently used. Although more sophisticated tensor estimation algorithms have been developed and described, application of these methods in neonatal diffusion tensor imaging studies seems to be low. More robust tensor estimation methods that exclude motion-corrupted directions prior to computation of the diffusion tensor generally require more processing time but can result in significantly improved data quality [27, 99].
A large portion of studies in this review used advanced post-processing methods such as tractography and tract-based spatial statistics. Diffusion tensor imaging data quality is of special importance in these methods. Insufficient diffusion tensor quality can result in early abortion of tracking streamlines or aberrant tract propagation and might have serious effects on reliability of final results. Use of tract-based spatial statistics, accurate spatial co-registration of different datasets is only achievable when slices are perfectly aligned in every dataset. Misalignment of slices caused by head motion during scanning might result in erroneous co-registration, affecting the reliability of the results. Sophisticated correction for misalignment and exclusion of incorrigible datasets prior to co-registration are therefore essential.
Future perspectives
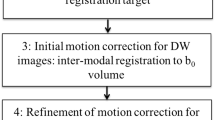
Ideally, MRI workstations should be equipped with state-of-the-art quality-checking software, with direct feedback during image acquisition. Such on-the-flight correction allows immediate re-scanning of slices that contain artefacts. Further refinement of these techniques might lead to significant improvements in data quality. Development of even more sophisticated diffusion tensor imaging acquisition schemes, implementation of higher-order processing algorithms in neonatal neuroimaging and further development of user-friendly software to detect and correct poor-quality datasets can result in significant improvements in data quality [26]. Furthermore, providing samples of actual diffusion data as Electronic supplementary material would be very useful to allow the readers to assess image quality. Furthermore, because alterations in myelination, water content and synaptogenesis result in rapidly changing diffusion characteristics within the first year of life, population-specific, standardized acquisition settings and processing pipelines of neonatal diffusion data are urgently needed (Fig. 2) [100, 101].
Overview of the processing pipeline for diffusion tensor imaging acquisition and analysis. Because all these steps determine data quality and analysis, reporting of these settings is valuable. Note: Outliers indicate motion-corrupted slices. FA fractional anisotropy, ROI regions of interest, TBSS tracts-based spatial statistics
Conclusion
Diffusion tensor imaging has great potential for investigation of the preterm brain provided that acquisition and post-processing pipelines are adapted to its specific characteristics. Current clinical studies pay little attention to this methodological requirement. In order to make bigger steps forward in understanding preterm brain structure, development and injury mechanisms, maximal awareness of these matters is required.
References
Gulland A (2012) Fifteen million and rising — the number of premature births every year. BMJ 344:e3084
Bhutta AT, Cleves MA, Casey PH et al (2002) Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA 288:728–737
Marlow N, Hennessy EM, Bracewell MA et al (2007) Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics 120:793–804
Rutherford MA, Supramaniam V, Ederies A et al (2010) Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology 52:505–521
Dyet LE, Kennea N, Counsell SJ et al (2006) Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics 118:536–548
Volpe JJ (2009) Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 8:110–124
Counsell SJ, Allsop JM, Harrison MC et al (2003) Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics 112:1–7
Dubois J, Dehaene-Lambertz G, Kulikova S et al (2014) The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience 276:48–71
Jones DK (2011) Diffusion MRI — theory, methods and applications. Oxford University Press USA, New York
Johansen-Berg H, Behrens TEJ (2014) Diffusion MRI — from quantitative measurement to in vivo neuroanatomy. Elsevier, London
Bassi L, Chew A, Merchant N (2011) Diffusion tensor imaging in preterm infants with punctate white matter lesions. Pediatr Res 69:561–566
Ment LR, Hirtz D, Huppi PS (2009) Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol 8:1042–1055
Tournier JD, Mori S, Leemans A (2011) Diffusion tensor imaging and beyond. Magn Reson Med 65:1532–1556
Ball G, Boardman JP, Aljabar P et al (2013) The influence of preterm birth on the developing thalamocortical connectome. Cortex 49:1711–1721
Bassi L, Ricci D, Volzone A et al (2008) Probabilistic diffusion tractography of the optic radiations and visual function in preterm infants at term equivalent age. Brain 131:573–582
de Bruine FT, van Wezel-Meijler G, Leijser LM et al (2011) Tractography of developing white matter of the internal capsule and corpus callosum in very preterm infants. Eur Radiol 21:538–547
van Kooij BJ, de Vries LS, Ball G et al (2012) Neonatal tract-based spatial statistics findings and outcome in preterm infants. AJNR Am J Neuroradiol 33:188–194
Dudink J, Kerr JL, Paterson K et al (2008) Connecting the developing preterm brain. Early Hum Dev 84:777–782
Pandit AS, Robinson E, Aljabar P et al (2014) Whole-brain mapping of structural connectivity in infants reveals altered connection strength associated with growth and preterm birth. Cereb Cortex 24:2324–2333
Collin G, van den Heuvel MP (2013) The ontogeny of the human connectome: development and dynamic changes of brain connectivity across the life span. Neuroscientist 19:616–628
Tymofiyeva O, Hess CP, Ziv E et al (2013) A DTI-based template-free cortical connectome study of brain maturation. PLoS One 8:e63310
Jones DK, Cercignani M (2010) Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed 23:803–820
Vos SB, Jones DK, Viergever MA et al (2011) Partial volume effect as a hidden covariate in DTI analyses. Neuroimage 55:1566–1576
Szczepankiewicz F, Latt J, Wirestam R et al (2013) Variability in diffusion kurtosis imaging: impact on study design, statistical power and interpretation. Neuroimage 76:145–154
Mathur AM, Neil JJ, McKinstry RC et al (2008) Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol 38:260–264
Kozak LR, David S, Rudas G et al (2013) Investigating the need of triggering the acquisition for infant diffusion MRI: a quantitative study including bootstrap statistics. Neuroimage 69:198–205
Plaisier A, Pieterman K, Lequin MH et al (2014) Choice of diffusion tensor estimation approach affects fiber tractography of the fornix in preterm brain. AJNR Am J Neuroradiol 35:1219–122528
Adams E, Chau V, Poskitt KJ et al (2010) Tractography-based quantitation of corticospinal tract development in premature newborns. J Pediatr 156:882–888
Aeby A, van Bogaert P, David P et al (2012) Nonlinear microstructural changes in the right superior temporal sulcus and lateral occipitotemporal gyrus between 35 and 43 weeks in the preterm brain. Neuroimage 63:104–110
Aeby A, de Tiege X, Creuzil M et al (2013) Language development at 2 years is correlated to brain microstructure in the left superior temporal gyrus at term equivalent age: a diffusion tensor imaging study. Neuroimage 78:145–151
Als H, Duffy FH, McAnulty GB et al (2004) Early experience alters brain function and structure. Pediatrics 113:846–857
Anjari M, Srinivasan L, Allsop JM et al (2007) Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. Neuroimage 35:1021–1027
Anjari M, Counsell SJ, Srinivasan L et al (2009) The association of lung disease with cerebral white matter abnormalities in preterm infants. Pediatrics 124:268–276
Arichi T, Moraux A, Melendez A et al (2010) Somatosensory cortical activation identified by functional MRI in preterm and term infants. Neuroimage 49:2063–2071
Arzoumanian Y, Mirmiran M, Barnes PD et al (2003) Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR Am J Neuroradiol 24:1646–1653
Ball G, Boardman JP, Arichi T et al (2013) Testing the sensitivity of tract-based spatial statistics to simulated treatment effects in preterm neonates. PLoS One 8:e67706
Ball G, Counsell SJ, Anjari M et al (2010) An optimised tract-based spatial statistics protocol for neonates: applications to prematurity and chronic lung disease. Neuroimage 53:94–102
Ball G, Boardman JP, Rueckert D et al (2012) The effect of preterm birth on thalamic and cortical development. Cereb Cortex 22:1016–1024
Berman JI, Mukherjee P, Partridge SC et al (2005) Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage 27:862–871
Berman JI, Glass HC, Miller SP et al (2009) Quantitative fiber tracking analysis of the optic radiation correlated with visual performance in premature newborns. AJNR Am J Neuroradiol 30:120–124
Bonifacio SL, Glass HC, Chau V et al (2010) Extreme premature birth is not associated with impaired development of brain microstructure. J Pediatr 157:726–732
Brummelte S, Grunau RE, Chau V et al (2012) Procedural pain and brain development in premature newborns. Ann Neurol 71:385–396
Chau V, Poskitt KJ, McFadden DE et al (2009) Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol 66:155–164
Chau V, Brant R, Poskitt KJ et al (2012) Postnatal infection is associated with widespread abnormalities of brain development in premature newborns. Pediatr Res 71:274–279
Cheong JLY, Thompson DK, Wang HX et al (2009) Abnormal white matter signal on MR imaging is related to abnormal tissue microstructure. AJNR Am J Neuroradiol 30:623–628
Counsell SJ, Shen Y, Boardman JP et al (2006) Axial and radial diffusivity in preterm infants who have diffuse white matter changes on magnetic resonance imaging at term-equivalent age. Pediatrics 117:376–386
de Bruine FT, van Wezel-Meijler G, Leijser LM et al (2013) Tractography of white-matter tracts in very preterm infants: a 2-year follow-up study. Dev Med Child Neurol 55:427–433
DeIpolyi AR, Mukherjee P, Gill K et al (2005) Comparing microstructural and macrostructural development of the cerebral cortex in premature newborns: diffusion tensor imaging versus cortical gyration. Neuroimage 27:579–586
Drobyshevsky A, Bregman J, Storey P et al (2007) Serial diffusion tensor imaging detects white matter changes that correlate with motor outcome in premature infants. Dev Neurosci 29:289–301
Dudink J, Lequin M, van Pul C et al (2007) Fractional anisotropy in white matter tracts of very-low-birth-weight infants. Pediatr Radiol 37:1216–1223
Dudink J, Larkman DJ, Kapellou O et al (2008) High b-value diffusion tensor imaging of the neonatal brain at 3T. AJNR Am J Neuroradiol 29:1966–1972
Dudink J, Buijs J, Govaert P et al (2010) Diffusion tensor imaging of the cortical plate and subplate in very-low-birth-weight infants. Pediatr Radiol 40:1397–1404
Gimenez M, Miranda MJ, Born AP et al (2008) Accelerated cerebral white matter development in preterm infants: a voxel-based morphometry study with diffusion tensor MR imaging. Neuroimage 41:728–734
Glass HC, Berman JI, Norcia AM et al (2010) Quantitative fiber tracking of the optic radiation is correlated with visual-evoked potential amplitude in preterm infants. AJNR Am J Neuroradiol 31:1424–1429
Groppo M, Ricci D, Bassi L et al (2014) Development of the optic radiations and visual function after premature birth. Cortex 56:30–37
Hasegawa T, Yamada K, Morimoto M et al (2011) Development of corpus callosum in preterm infants is affected by the prematurity: in vivo assessment of diffusion tensor imaging at term-equivalent age. Pediatr Res 69:249–254
Hemels MA, Nijman J, Leemans A et al (2012) Cerebral white matter and neurodevelopment of preterm infants after coagulase-negative staphylococcal sepsis. Pediatr Crit Care Med 13:678–684
Jo HM, Cho HK, Jang SH et al (2012) A comparison of microstructural maturational changes of the corpus callosum in preterm and full-term children: a diffusion tensor imaging study. Neuroradiology 54:997–1005
Lee AY, Jang SH, Lee E et al (2013) Radiologic differences in white matter maturation between preterm and full-term infants: TBSS study. Pediatr Radiol 43:612–619
Lee EC, Kwatra NS, Vezina G et al (2013) White matter integrity on fractional anisotropy maps in encephalopathic neonates post hypothermia therapy with normal-appearing MR imaging. Pediatr Radiol 43:709–715
Lepomaki V, Paavilainen T, Matomaki J et al (2012) Effect of antenatal growth and prematurity on brain white matter: diffusion tensor study. Pediatr Radiol 42:692–698
Lepomaki V, Leppanen M, Matomaki J et al (2013) Preterm infants’ early growth and brain white matter maturation at term age. Pediatr Radiol 43:1357–1364
Lepomaki VK, Paavilainen TP, Hurme SAM et al (2012) Fractional anisotropy and mean diffusivity parameters of the brain white matter tracts in preterm infants: reproducibility of region-of-interest measurements. Pediatr Radiol 42:175–182
Lepomaki V, Matomaki J, Lapinleimu H et al (2013) Effect of antenatal growth on brain white matter maturation in preterm infants at term using tract-based spatial statistics. Pediatr Radiol 43:80–85
Ling X, Tang W, Liu G et al (2013) Assessment of brain maturation in the preterm infants using diffusion tensor imaging (DTI) and enhanced T2 star weighted angiography (ESWAN). Eur J Radiol 82:e476–483
Liu Y, Baleriaux D, Kavec M et al (2010) Structural asymmetries in motor and language networks in a population of healthy preterm neonates at term equivalent age: a diffusion tensor imaging and probabilistic tractography study. Neuroimage 51:783–788
Liu Y, Metens T, Absil J et al (2011) Gender differences in language and motor-related fibers in a population of healthy preterm neonates at term-equivalent age: a diffusion tensor and probabilistic tractography study. AJNR Am J Neuroradiol 32:2011–2016
Liu Y, Aeby A, Baleriaux D et al (2012) White matter abnormalities are related to microstructural changes in preterm neonates at term-equivalent age: a diffusion tensor imaging and probabilistic tractography study. AJNR Am J Neuroradiol 33:839–845
Maas LC, Mukherjee P, Carballido-Gamio J et al (2004) Early laminar organization of the human cerebrum demonstrated with diffusion tensor imaging in extremely premature infants. Neuroimage 22:1134–1140
Mathew P, Pannek K, Snow P et al (2013) Maturation of corpus callosum anterior midbody is associated with neonatal motor function in eight preterm-born infants. Neural Plast 2013:359532
Melbourne A, Kendall GS, Cardoso MJ et al (2012) Radial structure in the preterm cortex: persistence of the preterm phenotype at term equivalent age? Med Image Comput Comput Assist Interv 15:256–263
Milgrom J, Newnham C, Anderson PJ et al (2010) Early sensitivity training for parents of preterm infants: impact on the developing brain. Pediatr Res 67:330–335
Nijman J, Gunkel J, de Vries LS et al (2013) Reduced occipital fractional anisotropy on cerebral diffusion tensor imaging in preterm infants with postnatally acquired cytomegalovirus infection. Neonatology 104:143–150
Nossin-Manor R, Card D, Morris D et al (2013) Quantitative MRI in the very preterm brain: assessing tissue organization and myelination using magnetization transfer, diffusion tensor and T1 imaging. Neuroimage 64:505–516
Paquette LB, Wisnowski JL, Ceschin R et al (2013) Abnormal cerebral microstructure in premature neonates with congenital heart disease. AJNR Am J Neuroradiol 34:2026–2033
Partridge SC, Mukherjee P, Henry RG et al (2004) Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. Neuroimage 22:1302–1314
Partridge SC, Mukherjee P, Berman JI et al (2005) Tractography-based quantitation of diffusion tensor imaging parameters in white matter tracts of preterm newborns. J Magn Reson Imaging 22:467–474
Pogribna U, Yu X, Burson K et al (2013) Perinatal clinical antecedents of white matter microstructural abnormalities on diffusion tensor imaging in extremely preterm infants. PLoS One 8:e72974
Pogribna U, Burson K, Lasky RE et al (2014) Role of diffusion tensor imaging as an independent predictor of cognitive and language development in extremely low-birth-weight infants. AJNR Am J Neuroradiol 35:790–796
Ratnarajah N, Rifkin-Graboi A, Fortier MV et al (2013) Structural connectivity asymmetry in the neonatal brain. Neuroimage 75:187–194
Reiman M, Parkkola R, Johansson R et al (2009) Diffusion tensor imaging of the inferior colliculus and brainstem auditory-evoked potentials in preterm infants. Pediatr Radiol 39:804–809
Rogers CE, Anderson PJ, Thompson DK et al (2012) Regional cerebral development at term relates to school-age social-emotional development in very preterm children. J Am Acad Child Adolesc Psychiatry 51:181–191
Rose SE, Hatzigeorgiou X, Strudwick MW et al (2008) Altered white matter diffusion anisotropy in normal and preterm infants at term-equivalent age. Magn Reson Med 60:761–767
Rose J, Butler EE, Lamont LE et al (2009) Neonatal brain structure on MRI and diffusion tensor imaging, sex, and neurodevelopment in very-low-birthweight preterm children. Dev Med Child Neurol 51:526–535
Shim SY, Jeong HJ, Son DW et al (2012) Altered microstructure of white matter except the corpus callosum is independent of prematurity. Neonatology 102:309–315
Skiold B, Horsch S, Hallberg B et al (2010) White matter changes in extremely preterm infants, a population-based diffusion tensor imaging study. Acta Paediatr Int J Paediatr 99:842–849
Tam EWY, Ferriero DM, Xu D et al (2009) Cerebellar development in the preterm neonate: effect of supratentorial brain injury. Pediatr Res 66:102–106
Thompson DK, Inder TE, Faggian N et al (2011) Characterization of the corpus callosum in very preterm and full-term infants utilizing MRI. Neuroimage 55:479–490
Thompson DK, Inder TE, Faggian N et al (2012) Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. Neuroimage 59:3571–3581
van Kooij BJ, van Pul C, Benders MJ et al (2011) Fiber tracking at term displays gender differences regarding cognitive and motor outcome at 2 years of age in preterm infants. Pediatr Res 70:626–632
van Pul C, van Kooij BJ, de Vries LS et al (2012) Quantitative fiber tracking in the corpus callosum and internal capsule reveals microstructural abnormalities in preterm infants at term-equivalent age. AJNR Am J Neuroradiol 33:678–684
Vinall J, Grunau RE, Brant R et al. (2013) Slower postnatal growth is associated with delayed cerebral cortical maturation in preterm newborns. Sci Transl Med 5:168ra168
Yoo SS, Park HJ, Soul JS et al (2005) In vivo visualization of white matter fiber tracts of preterm- and term-infant brains with diffusion tensor magnetic resonance imaging. Invest Radiol 40:110–115
Zwicker JG, Grunau RE, Adams E et al (2013) Score for neonatal acute physiology-II and neonatal pain predict corticospinal tract development in premature newborns. Pediatr Neurol 48:123–129
Keil B, Alagappan V, Mareyam A et al (2011) Size-optimized 32-channel brain arrays for 3 T pediatric imaging. Magn Reson Med 66:1777–1787
Pannek K, Guzzetta A, Colditz PB et al (2012) Diffusion MRI of the neonate brain: acquisition, processing and analysis techniques. Pediatr Radiol 42:1169–1182
Jones DK, Leemans A (2011) Diffusion tensor imaging. Methods Mol Biol 711:127–144
Veraart J, Rajan J, Peeters RR et al (2013) Comprehensive framework for accurate diffusion MRI parameter estimation. Magn Reson Med 70:972–984
Chang LC, Jones DK, Pierpaoli C (2005) RESTORE: robust estimation of tensors by outlier rejection. Magn Reson Med 53:1088–1095
Plaisier A, Govaert P, Lequin MH et al (2014) Optimal timing of cerebral MRI in preterm infants to predict long-term neurodevelopmental outcome: a systematic review. AJNR Am J Neuroradiol 35:841–847
Pannek K, Scheck SM, Colditz PB et al (2014) Magnetic resonance diffusion tractography of the preterm infant brain: a systematic review. Dev Med Child Neurol 56:113–124
Acknowledgments
The research of K. Pieterman is supported by a Ter Meulen Grant of the Royal Netherlands Academy of Arts and Sciences (KNAW). The research of A. Leemans is supported by VIDI Grant 639.072.411 from the Netherlands Organisation for Scientific Research (NWO).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 30 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Pieterman, K., Plaisier, A., Govaert, P. et al. Data quality in diffusion tensor imaging studies of the preterm brain: a systematic review. Pediatr Radiol 45, 1372–1381 (2015). https://doi.org/10.1007/s00247-015-3307-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-015-3307-y