Abstract
Background
The sonographic (US) features of limb–body wall complex have been well documented; however the literature regarding the findings on MRI in limb–body wall complex is scant.
Objective
To characterize the prenatal MRI features of limb–body wall complex.
Materials and methods
We performed a retrospective review of all MRI scans of fetuses diagnosed with limb–body wall complex at our institution from 2001 to 2011. Fetuses without correlating US scans or follow-up information were excluded. Three pediatric radiologists blinded to the specific US findings reviewed the prenatal MRIs. Images were evaluated for the organ location and attachment, the body part affected, characterization of the body wall defect, and spinal, limb and umbilical cord abnormalities.
Results
Ten subjects met inclusion criteria. MRI was able to detect and characterize the body part affected and associated abnormalities. All fetuses had ventral wall defects, a small thorax and herniated liver and bowel. The kidneys were extracorporeal in three cases. The extruded organs were attached to the placenta or the uterine wall in all cases. Abnormal spinal curvatures of various degrees of severity were present in all cases. Eight cases had a short, uncoiled cord. Limb anomalies were present in 6 of the 10 cases.
Conclusion
We illustrate the common fetal MRI findings of limb–body wall complex. The prenatal diagnosis of limb–body wall complex and the differentiation of this defect from treatable abdominal wall defects are crucial to providing appropriate guidance for patient counseling and management.
Similar content being viewed by others
Introduction
Limb–body wall complex, also known as body stalk anomaly, amniotic band disruption complex, and amnion rupture sequence [1–4], refers to a severe pattern of fetal polymalformation characterized by Van Allen et al. [5] by the presence of at least two of the following three major anomalies: (1) thoraco or abdominoschisis, (2) limb defects and (3) exencephaly or encephalocele with facial defects. However in the prenatal US literature limb–body wall complex is typified by the presence of a major abdominal wall defect, abnormal spinal curvatures and the presence of a rudimentary umbilical cord [6–8]. The diagnosis of limb–body wall complex can usually be made on prenatal US by the end of the first trimester [6, 9, 10].
Limb–body wall complex is primarily differentiated from other entities with abdominal wall defects based on the position of the defect with respect to the umbilical cord insertion. The ventral wall defect in limb–body wall complex commonly consists of a large, eccentric, lateral abdominal wall defect with the abdominal organs directly attached to the placenta or the uterine wall [5, 10]. In addition to the type of defect, the size of the defect and the presence of associated anomalies help to establish the correct diagnosis on sonography [10, 11]. Associated reported malformations include maldevelopment of the hindgut; ectopia cordis; neural tube defects; scoliosis, kyphoscoliosis and spinal anomalies; visceral malformations; the persistence of the extra-embryonic coelomic cavity; enlarged nuchal translucency measurements; increased maternal serum alpha-fetoprotein (AFP); a single umbilical artery; and the presence of a short or malformed, rudimentary umbilical cord [3, 6–8, 12–16]. The fetal karyotype is usually normal [5, 10, 11].
According to the literature, limb–body wall complex can present along a spectrum of variable phenotypes [4] that can have different underlying etiologies. Two main distinctive phenotypes of limb–body wall complex have been described [7, 12, 17–19]: type 1, or placento-cranial phenotype, presents with predominantly craniofacial and upper limb abnormalities, and cranial placental attachment; type 2, or placento-abdominal phenotype, is characterized by placento-abdominal attachments and no associated craniofacial defects [12, 17, 19]. The type 2 form of limb–body wall complex is often associated with a short umbilical cord, severe urogenital anomalies and anal atresia from the persistence of the primitive cloaca, and lumbosacral meningocele, in addition to placental abnormalities characterized by an intact amnion and a short umbilical cord; furthermore the lower half of the fetal body is found within the unobliterated extra-embryonic coelomic cavity [12, 19].
A prenatal diagnosis of limb–body wall complex and its differentiation from other potentially treatable abdominal wall defects is crucial because the prognosis for limb–body wall complex is uniformly poor. There are no currently available fetal interventions and there is nearly universal early postnatal death [3, 6, 7, 10, 20, 21]. In addition, Costa et al. [10] recently reported an increased risk of maternal morbidity and adverse maternal outcomes in women with late diagnosis or who choose not to terminate pregnancy. In these instances, complications in labor and cesarean section can occur, including uterine rupture. Costa et al. [10] emphasized that these points should be discussed during counseling and that termination of pregnancy should be advised when a definitive diagnosis of limb–body wall complex is made.
Antenatal US is an established diagnostic tool to identify the features of limb–body wall complex and the diagnosis of limb–body wall complex is usually not difficult to make [3, 19, 22–24]. However in cases of severe oligohydramnios or large maternal body habitus and in cases in which an unusually complex relationship between the herniated viscera and the deformities exists, the diagnosis of limb–body wall complex can pose a challenge [25]. In addition, given the lethality of the condition and the increased risk of maternal morbidity and adverse maternal outcomes, it may be of value to referring physicians to have an additional diagnostic modality to provide appropriate counseling in these difficult situations.
The aim of this study was to analyze a cohort of fetuses with limb–body wall complex who were examined with MRI at our institution during a 10-year period and to identify relevant MRI findings that characterize this condition. It was also our intent to report whether MR offers another noninvasive and reliable method of imaging the features of limb–body wall complex that involve the spine, limbs, cranium, internal organs, placenta and umbilical cord.
Materials and methods
A retrospective review of limb–body wall complex cases diagnosed between January 2001 and December 2011 was performed using the database at the Center for Fetal Diagnosis and Treatment at Children’s Hospital of Philadelphia. This study was approved by our institutional review board, and written consent of the patients was waived.
It is our routine practice to perform fetal MRI the same day as a level III US exam in cases of abdominal wall defects with the exception of gastroschisis. The inclusion criteria for the study were the presence of thoracoabdominal wall defects, spinal abnormalities or limb anomalies. Cases were excluded if the final clinical or imaging diagnosis was not limb–body wall complex, if no uterine or placental attachments were present, if a concomitant US result was not available for review or if follow-up information was not available.
MRI exams were performed with a Siemens 1.5-tesla scanner (Magnetom Avanto and Symphony; Siemens, Erlangen, Germany) using a torso phased-array coil. Sequences performed were half-Fourier acquisition single-shot turbo spin-echo (HASTE), T2-weighted steady-state free precession (TruFisp, true fast imaging with steady-state precession), T1-weighted fast low-angle shot (FLASH) and echo planar imaging (EPI) in at least two planes. Parameters included a matrix of 256 × 256 and a field-of-view of 30 cm or less when feasible. Slice thickness for all sequences ranged from 3 mm to 5 mm with no gap.
All MRI exams were initially reviewed by three pediatric radiologists, in conference, who had 10 (AMJ), 6 (ME), and 5 (TV) years of experience in fetal imaging and who analyzed the contents of the abdominal wall defect, the organ location and attachment, spinal anomalies, the umbilical cord and limb anomalies. Although the reviewers were blinded to the specific US and MRI findings, they were aware that all the fetuses carried a diagnosis of limb–body wall complex. A radiology resident (EAP) and a pediatric radiologist (TV) then reviewed the US reports, tabulated the data, compared the MRI findings to the data found in each patient’s medical record and categorized the findings.
Results
A total of 18 cases of limb–body wall complex were examined with MRI. Five cases were excluded for lack of placental or uterine attachments. Three additional cases were excluded for lack of correlating US data. The mean gestational age was 20 weeks (range 17–24 weeks) and mean maternal age was 27 years (range 23–33 years) (Table 1).
The main findings were organized according to the following categories: thoracoabdominal wall defect, limb anomalies, spinal anomalies, the umbilical cord, craniofacial defects, and internal malformations.
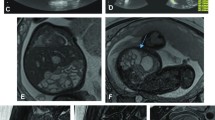
Thoracoabdominal wall defects (Fig. 1)
Thoracoabdominal wall defects in a 22-week fetus (patient 5) with findings of omphalocele and limb anomalies on US. a–c Parasagittal T2-weighted MRI images show marked scoliosis with 90-degree angulation. Abdominal contents lie in an extracorporeal location, including liver (L), bowel (B), stomach (S) and kidney (white arrow). Extruded bowel (B) loops and liver (L) are seen attached to the uterine wall (black arrows). The limbs, although abnormally positioned, appear normal
All ten fetuses had large thoracoabdominal wall defects and correlating hypoplastic thoraces with extruded organs in 100% of the MR scans. All exhibited herniated liver and bowel loops. The stomach was extra-abdominal in all fetuses (100%). In three cases (30%) the kidneys were in an extracorporeal location. A portion of the bladder was extra-abdominal in one case (10%) and was not identified in three fetuses (30%). The heart was partially extruded in four cases (40%).
We evaluated whether there was a membrane covering the defect and the attachment of the extruded organs to the placenta or the uterine wall, and there was no membrane covering the defect in any of the cases. In all cases (100%), the MRI scans revealed organs attached to the placenta or uterine wall (consistently bowel loops and additionally the liver in five cases).
Limb anomalies (Figs. 2, 3 and 4)
Limb and other anomalies in an 18-week-old fetus (patient 10) with concern for sacrococcygeal teratoma on routine prenatal US referred to level III US and MRI for further workup. a, b US images show abnormal orientation of the right lower extremity. Asterisks denote the spine. There is an abdominal wall defect with herniation of the entire liver (L), gallbladder (GB) and bowel (B), which is attached to uterine wall (black arrows). H head, RT foot, right foot. c, d Correlative sagittal T2-weighted MR images show similar findings with a defect of the anterior abdominal wall. The urinary bladder is not identified. The right lower extremity (arrows) appears hypoplastic and is abnormally flexed and located posterior to the body, with the foot pointed toward the head. The bony pelvis is not formed and scoliosis is noted. Upper extremities were normal. Arrowhead denotes one of the hands, with five digits
Abnormal extremities in a 19-week fetus (patient 4) and abdominal wall defect on US. a, b Sagittal T2-weighted and (c) sagittal T1-weighted MR images show an abnormally positioned lower limb (arrowheads). The liver (L) and a portion of the bowel (B) are extruded. Note the high T1 signal intensity of the liver and meconium in (c)
Limb anomalies were present in 6 of 10 cases (60%). The most frequent anomaly was an abnormal limb position, in 5 of 10 fetuses (50%). One of the lower extremities was absent in 2 of the 10 fetuses (20%).
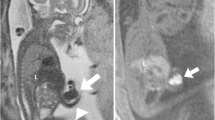
Spinal anomalies (Fig. 5)
Anomalies in a 24-week fetus (patient 9) with severe scoliotic deformity. a, b Three-dimensional volume-rendered US images show acute-angle curvature of the spine in the thoracolumbar region. c Coronal T2-weighted MR image shows a large anterolateral abdominal wall defect with extruded stomach (S), bowel (B) and liver (L) attached to the uterine wall (arrows). Note the abnormal orientation of a lower limb (arrowheads). There is no membrane covering the extra-abdominal organs
Various degrees of thoracolumbar scoliosis/kyphoscoliosis were noted in all cases, and this defect was graded as severe (near 90° of angulation) in 6 of 10 cases (60%). In three cases the MRI scans revealed diastematomyelia (30%).
Umbilical cord (Fig. 6)
Umbilical cord anomalies in a 22-week fetus (patient 5) with limb–body wall complex. Parasagittal T2-weighted MR image shows a very short, uncoiled, two-vessel cord (arrows). Its insertion is immediately over the abdominal wall defect. Cord length depends on the tension caused by the fetal movements. When the umbilical cord is short, it is associated with fetal immobility. Coiling can be seen at about 6–9 weeks and it is usually counter-clockwise. Lack of coiling is sometimes the result of fetal akinesia and should raise concern for congenital anomalies
Six of the ten cases (60%) had a subjectively short and uncoiled two-vessel cord. In two cases (20%) the MRI scans revealed a three-vessel, normal-length coiled cord. A short uncoiled cord but with the expected three vessels was seen in 2 of the 10 fetuses (20%).
Craniofacial defects
At our institution fetal MRI studies can be performed as dedicated fetal body exams or as dedicated fetal central nervous system (CNS) studies. Fetal body studies are interpreted by pediatric body radiologists and are ordered when pathology in the fetal body is suspected, typically on previous US studies. Fetal CNS exams consist of studies tailored for detailed evaluation of the fetal CNS. Fetal CNS exams are ordered when fetal head and neck pathology is suspected, and these studies are interpreted by pediatric neuroradiologists. Six of the fetuses were evaluated with complete fetal body and fetal CNS exams. However, in 4 of the 10 fetuses the MRI examinations were not tailored for CNS evaluation; therefore the craniofacial structures were difficult to assess retrospectively in these four cases, although craniofacial defects were clearly excluded on US in all cases. Of the six fetuses with fetal body and fetal CNS exams, only one patient demonstrated the stigmata of Chiari II malformation, which was correctly identified in both modalities.
Discussion
This is a unique case series focused on the description of the prenatal MRI findings of limb–body wall complex. Several case series and reports of limb–body wall complex imaged with US have been reported [6, 19, 22–24, 26]; however none has provided a detailed description of the MRI findings. Furthermore there is only one case report describing the use of MRI in the diagnosis of limb–body wall complex [27].
Limb–body wall complex is almost uniformly lethal with a very poor outcome due to, among other factors, pulmonary hypoplasia [11, 28, 29]. The commonly quoted prevalence of 1:14,000–1:42,000 pregnancies [3, 10, 11, 30, 31] probably underestimates the true prevalence of limb–body wall complex, possibly because of the high incidence of fetal miscarriage with this defect and the increasing use of first-trimester US screening [3, 10]. Daskalakis et al. [6] recently reported a prevalence of about 1:7,500 pregnancies at 10–14 weeks of gestation.
The precise etiopathogenesis of limb–body wall complex remains uncertain, with several competing pathophysiological theories. In the amniotic band theory [1, 32], limb–body wall complex is believed to be the result of an early rupture of the amnion that results in bands entrapping various parts of the fetus and disturbing normal development. In this theory the variable phenotypes are thought to be related to the timing of the amniotic rupture [1]; however this theory could not explain the internal abnormalities observed in some of the cases, such as the absence of a lung or a kidney. Van Allen et al. [5, 24] later proposed that the observed abnormalities are related to vascular impairment in the 4th through the 6th weeks of gestation, resulting in disruptive and structural defects. Others have proposed that this sequence of abnormalities is the result of a teratogenic exposure during early gestation [33] or of a primary ectodermal defect of the early embryonic disc [4]. One of the most accepted theories is that of a very early embryonic maldevelopment secondary to a faulty embryonic folding process [3, 6, 12, 19, 34, 35] that is possibly related to a malfunction of the ectodermal placodes [19, 35].
All of the cases in our series were of the placento-abdominal phenotype, or type 2, and in all of our cases attachments of the extruded viscera to the placenta or the uterine wall could be easily demonstrated on MRI. None of the fetuses exhibited exencephaly, encephalocele or facial clefts in their level III US or in their MRI exams. We presume this might be related to the fact that type 1, or placento-cranial phenotype, cases may be diagnosed as early as the 10th to 14th weeks of pregnancy [6], which would be too early to perform fetal MRI exams and would potentially result in termination of pregnancy or a spontaneous abortion. In addition, in the experience of Chen et al. [7] the diagnosis of type 1 limb–body wall complex is less common than the diagnosis of type 2 limb–body wall complex, with a reported incidence of 1 in 28,000 births for type 1 versus 1 in 9,400 for type 2.
Encephalic anomalies in limb–body wall complex are usually severe and are typically associated with the type 1 phenotype. The reported abnormalities include exencephaly or encephalocele with or without associated facial defects [1, 35]. In our case series none of the fetuses demonstrated these abnormalities, supporting the hypothesis that these two phenotypes have a different underlying etiology and are morphologically and possibly pathogenetically different [12]. Furthermore, the coexistence of both phenotypes has been anecdotally observed [12, 26]. Alternatively, and because fetal MRI is typically performed after the 20th week, this low incidence might be in part related to the increased lethality and early pregnancy loss in limb–body wall complex cases with craniofacial abnormalities [7]. Other authors have disagreed with this dichotomous distinction, because cases with concomitant craniofacial and genital anomalies have been described [4, 5].
Skeletal anomalies are frequently described in limb–body wall complex series, including limb and spinal anomalies. Limb anomalies have been accepted as an inclusion criterion [24], but these anomalies are variable in type and severity and seem to be etiologically heterogeneous. Limb anomalies include reduction defects such as amelia, hypoplasia and positional deformations from clubfoot to limb malrotation often associated with hemipelvis. In our series, no upper limb reduction defects were present and only abnormalities in orientation were noted in the upper extremities of 2 of the 10 fetuses, both of which had abnormal orientation of all four extremities, which was less frequent in our study than described in the literature [5, 19, 24, 35]. Upper limb anomalies are also more frequently seen in association with placento-cranial adhesions, typically in the setting of type 1 limb–body wall complex [7]. We more commonly found lower extremity anomalies, as expected from the literature [7, 19, 23, 24, 35]. It has been reported that cases of limb–body wall complex without craniofacial abnormalities, those conforming to the type 2 placento-abdominal phenotype, usually manifest with lower limb anomalies, placenta–abdominal attachment, visceral anomalies (abnormal genitalia, anal atresia, renal defects), the persistence of the extra-embryonic coelom, and umbilical cord abnormalities [7, 36], as noted in our series.
Spinal anomalies in limb–body wall complex include severe scoliosis and dysraphisms. It is thought that aplasia or hypoplasia of the paraspinous or thoracolumbar musculature might be responsible in part for the severe scoliosis [11, 24] and is more commonly encountered in type 2 limb–body wall complex [12, 19].
All of our patients showed extensive thoracoabdominal defects, which is in line with previous studies [6, 12, 19, 22]. A membrane covering the defect could not be identified in any of our cases. This membrane is thought to be made of amnion and peritoneal layers and is sometimes attached to the placenta or the uterine wall [37]. Visceral anomalies are also frequently associated with limb–body wall complex, including malrotation-like anomalies from the abdominal wall defect and atresias secondary to a compromised vasculature. Cardiac anomalies, lung hypoplasia and renal agenesis have also been described [5].
The differential diagnoses of severe ventral wall anomalies without craniofacial defects include omphalocele and pentalogy of Cantrell, both of which usually consist of a midline defect at the umbilical cord insertion; gastroschisis, characteristically described as a right, small paraumbilical defect; bladder and cloacal exstrophy, characterized by defects in the infraumbilical location; and omphalocele-exstrophy-imperforate anus-spinal defects complex (OEIS) [3, 20]. However the defects in limb–body wall complex are the only defects characteristically associated with scoliosis, which typically suggests the diagnosis [6, 7]. Moderate to severe scoliotic deformities were noted in 9 of our 10 cases with angles of approximately 90° appreciated in 60% of the fetuses. The presence of severe scoliosis is an important finding that should increase the level of suspicion for a diagnosis of limb–body wall complex [7]. Three cases of limb–body wall complex were initially misdiagnosed at our institution, which is not unexpected because pediatric radiologists may be unfamiliar with this condition and its appearance on MRI. Given the increased use of fetal imaging, pediatric radiologists are in a pivotal position to recognize this diagnosis, and awareness of the MR imaging features of limb–body wall complex is crucial given the lethality of the condition and the important implications in management. Furthermore, in cases in which the diagnosis of limb–body wall complex is indisputable and considering that limb–body wall complex is a sporadic anomaly that is not typically associated with chromosomal abnormalities, karyotype testing could be obviated. Given the poor prognosis in this entity, emphasis should be placed on maternal safety and potential pregnancy complications [10, 17, 21].
Examination of the umbilical cord might provide additional clues to support a diagnosis of limb–body wall complex. The normal umbilical cord contains two arteries and one vein, which are wound along the cord’s central axis to form a twisted appearance. Coiling is seen at approximately 6–9 weeks, and it is usually counter-clockwise [38]. The average length of the umbilical cord is 55 cm [39, 40]. Its length depends on the tension caused by the fetal movements. When the umbilical cord is short or uncoiled, it is usually associated with immobility of the fetus [41] and one should suspect congenital anomalies. This correlates well with our case series, in which 80% of the cases had an abnormal umbilical cord.
The strengths of our study include a relatively large cohort of patients from a tertiary referral center with a final diagnosis of limb–body wall complex. However, our study had several limitations, and the most important was its retrospective nature. A further limitation is that all of the fetuses evaluated in this study were of the type 2 placento-abdominal phenotype of limb–body wall complex. In addition, observer bias was present in our study because the observing radiologists were aware that the patients had limb–body wall complex. Finally, only the US reports were reviewed without the images. However, all of the US exams were interpreted by radiologists subspecializing in obstetric imaging and all of the studies were performed as level III US exams by highly experienced sonographers.
Conclusion
We report the common fetal MRI findings of limb–body wall complex as a complementary tool to sonography. Awareness and early, accurate recognition of this near uniformly fatal entity will help to differentiate limb–body wall complex from other more benign, potentially treatable abdominal wall defects in order to provide adequate guidance for parental counseling and management.
References
Higginbottom MC, Jones KL, Hall BD et al (1979) The amniotic band disruption complex: timing of amniotic rupture and variable spectra of consequent defects. J Pediatr 95:544–549
Kalousek DK, Bamforth S (1988) Amnion rupture sequence in previable fetuses. Am J Med Genet 31:63–73
Smrcek JM, Germer U, Krokowski M et al (2003) Prenatal ultrasound diagnosis and management of body stalk anomaly: analysis of nine singleton and two multiple pregnancies. Ultrasound Obstet Gynecol 21:322–328
Hunter AG, Seaver LH, Stevenson RE (2011) Limb–body wall defect. Is there a defensible hypothesis and can it explain all the associated anomalies? Am J Med Genet A 155:2045–2059
Van Allen MI, Curry C, Gallagher L (1987) Limb body wall complex: I. Pathogenesis. Am J Med Genet 28:529–548
Daskalakis G, Sebire NJ, Jurkovic D et al (1997) Body stalk anomaly at 10–14 weeks of gestation. Ultrasound Obstet Gynecol 10:416–418
Chen CP, Lin CJ, Chang TY et al (2007) Second-trimester diagnosis of limb–body wall complex with literature review of pathogenesis. Genet Couns 18:105–112
Goldstein I, Winn HN, Hobbins JC (1989) Prenatal diagnostic criteria for body stalk anomaly. Am J Perinatol 6:84–85
Shalev E, Eliyahu S, Battino S et al (1995) First trimester transvaginal sonographic diagnosis of body stalk anomaly (correction of anatomy). J Ultrasound Med 14:641–642
Costa ML, Couto E, Furlan E et al (2012) Body stalk anomaly: adverse maternal outcomes in a series of 21 cases. Prenat Diagn 32:264–267
Mann L, Ferguson-Smith MA, Desai M et al (1984) Prenatal assessment of anterior abdominal wall defects and their prognosis. Prenat Diagn 4:427–435
Russo R, Vecchione R (1996) Limb body wall complex: craniofacial defects as a distinctive factor. Birth Defects Orig Artic Ser 30:157–164
Negishi H, Yaegashi M, Kato EH et al (1998) Prenatal diagnosis of limb–body wall complex. J Reprod Med 43:659–664
Murphy A, Platt LD (2011) First-trimester diagnosis of body stalk anomaly using 2- and 3-dimensional sonography. J Ultrasound Med 30:1739–1743
Luehr B, Lipsett J, Quinlivan JA (2002) Limb–body wall complex: a case series. J Matern Fetal Neonatal Med 12:132–137
Levy R, Lacombe D, Rougier Y et al (2007) Limb body wall complex and amniotic band sequence in sibs. Am J Med Genet A 143:2682–2687
Colpaert C, Bogers J, Hertveldt K et al (2000) Limb–body wall complex: 4 new cases illustrating the importance of examining placenta and umbilical cord. Pathol Res Pract 196:783–790
Luebke HJ, Reiser CA, Pauli RM (1990) Fetal disruptions: assessment of frequency, heterogeneity, and embryologic mechanisms in a population referred to a community-based stillbirth assessment program. Am J Med Genet 36:56–72
Pumberger W, Schaller A, Bernaschek G (2001) Limb–body wall complex: a compound anomaly pattern in body-wall defects. Pediatr Surg Int 17:486–490
Emanuel PG, Garcia GI, Angtuaco TL (1995) Prenatal detection of anterior abdominal wall defects with US. Radiographics 15:517–530
Mathai AM, Menezes RG, Kumar S et al (2009) A fetal autopsy case of body stalk anomaly. Leg Med 11:241–244
Sahinoglu Z, Uludogan M, Arik H et al (2007) Prenatal ultrasonographical features of limb body wall complex: a review of etiopathogenesis and a new classification. Fetal Pediatr Pathol 26:135–151
Tang TT, Oechler HW, Hinke DH et al (1991) Limb body–wall complex in association with sirenomelia sequence. Am J Med Genet 41:21–25
Van Allen MI, Curry C, Walden CE et al (1987) Limb–body wall complex: II. Limb and spine defects. Am J Med Genet 28:549–565
Fukumasu H, Imanaka M, Matsumoto M et al (1993) Significance of fetography in prenatal diagnosis of limb–body wall complex (LBWC). Int J Gynaecol Obstet 43:317–321
Chikkannaiah P, Dhumale H, Kangle R et al (2013) Limb body wall complex: a rare anomaly. J Lab Physicians 5:65–67
Chen C-P, Cheng S-J, Lin Y-H et al (2005) Prenatal imaging of limb–body wall complex by magnetic resonance imaging. Prenat Diagn 25:521–523
De Silva MV, Senanayake H, Siriwardana KD (2001) Body stalk anomaly. Ceylon Med J 46:68
Kanamori Y, Hashizume K, Sugiyama M et al (2007) Long-term survival of a baby with body stalk anomaly: report of a case. Surg Today 37:30–33
Morrow RJ, Whittle MJ, McNay MB et al (1993) Prenatal diagnosis and management of anterior abdominal wall defects in the west of Scotland. Prenat Diagn 13:111–115
Forrester MB, Merz RD (1999) Epidemiology of abdominal wall defects, Hawaii, 1986–1997. Teratology 60:117–123
Torpin R (1965) Amniochorionic mesoblastic fibrous strings and amniotic bands: associated constricting fetal malformations or fetal death. Am J Obstet Gynecol 91:65–75
Herva R, Karkinen-Jääskeläinen M (1984) Amniotic adhesion malformation syndrome: fetal and placental pathology. Teratology 29:11–19
Duhamel B (1963) Embryology of exomphalos and allied malformations. Arch Dis Child 38:142–147
Hartwig NG, Vermeij-Keers C, De Vries HE et al (1989) Limb body wall malformation complex: an embryologic etiology? Hum Pathol 20:1071–1077
Deruelle P, Hay R, Subtil D et al (2000) [Antenatal diagnosis of limb body wall complex]. J Gynecol Obstet Biol Reprod 29:385–391
Lockwood CJ, Scioscia AL, Hobbins JC (1986) Congenital absence of the umbilical cord resulting from maldevelopment of embryonic body folding. Am J Obstet Gynecol 155:1049–1051
Kalish RB, Hunter T, Sharma G et al (2003) Clinical significance of the umbilical cord twist. Am J Obstet Gynecol 189:736–739
Ente G, Penzer PH (1991) The umbilical cord: normal parameters. J R Soc Health 111:138–140
Naeye RL (1985) Umbilical cord length: clinical significance. J Pediatr 107:278–281
Miller ME, Higginbottom M, Smith DW (1981) Short umbilical cord: its origin and relevance. Pediatrics 67:618–621
Acknowledgments
This work received the Caffey Award for Best Scientific Exhibit, Society for Pediatric Radiology, 55th Annual Meeting & Postgraduate Course 2012, San Francisco, CA.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguirre-Pascual, E., Epelman, M., Johnson, A.M. et al. Prenatal MRI evaluation of limb–body wall complex. Pediatr Radiol 44, 1412–1420 (2014). https://doi.org/10.1007/s00247-014-3026-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-014-3026-9