Abstract
Background
Studies have shown that the fracture plane of the classic metaphyseal lesion (CML) of infant abuse occurs in the region of the primary spongiosa, encompassing a radiodense fracture fragment customarily referred to as the “zone of provisional calcification” or ZPC. However, the zone of provisional calcification is defined differently in the pathology and the imaging literature, potentially impeding efforts to understand the fundamental morphological features of the classic metaphyseal lesion.
Objective
We systematically correlated micro-CT data with histology in piglets to explore the differing definitions of the zone of provisional calcification and to elucidate the anatomical basis for divergent definitions.
Materials and methods
The distal tibias of five normal fetal piglets were studied postmortem. The specimens were resected and imaged with digital radiography (50 μm resolution) and micro-CT (45 μm3 isotropic resolution). Image processing techniques were applied to the micro-CT data for visualization and data analysis. The resected tissue specimens were then processed routinely and the light microscopic features were correlated with the imaging findings.
Results
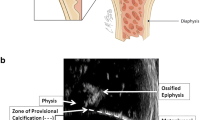
The longitudinal dimension of the radiologic zone of provisional calcification is greater than the histological ZPC, and these dimensions are statistically distinct (P < 0.0002). The radiologic zone of provisional calcification consists of two adjoining mineralized discoid regions that span the chondro-osseous junction—a thick discoid region that encompasses the densest region of the primary spongiosa, and a thin discoid region (corresponding to the histological ZPC) that is situated in the base of the physis adjacent to the metaphysis.
Conclusion
The correlation of the normal histology and micro-CT appearance of this dynamic and complex region provides an anatomical foundation upon which a deeper appreciation of the morphology of the classic metaphyseal lesion can be built.







Similar content being viewed by others
References
Kleinman PK, Marks S, Blackbourne B (1986) The metaphyseal lesion in abused infants: a radiologic histopathologic study. AJR Am J Roentgenol 146:896–905
Boal D (2001) Child abuse roundtable discussion: controversial aspects of child abuse: 43rd annual meeting, Society for Pediatric Radiology. Pediatr Radiol 31:760–774
Kleinman PK, Perez-Rossello JM, Newton AW et al (2011) Prevalence of the classic metaphyseal lesion in infants at low versus high risk for abuse. AJR Am J Roentgenol 197:1005–1008
Kleinman PK, Marks S (1996) A regional approach to classic metaphyseal lesions in abused infants: the distal tibia. AJR Am J Roentgenol 166:1207–1212
Kleinman PK (1998) Diagnostic imaging of child abuse, 2nd edn. Mosby-Year Book Inc., Philadelphia
Dodds GS, Cameron HC (1934) Studies on experimental rickets in rats. I. Structural modifications of the epiphyseal cartilages in the tibia and other bones. Am J Anat 55:135–165
McLean FC, Bloom W (1940) Calcification and ossification. Calcification in normal growing bone. Anat Rec 78:333–359
Caffey J (1945) Pediatric x-ray diagnosis. Year Book Medical Publishers, Chicago, pp 558, 561–562
Oestreich A (2003) The acrophysis: a unifying concept for enchondral bone growth and its disorders. I. Normal growth. Skeletal Radiol 32:121–127
Laor T, Jaramillo D (2009) MR imaging insights into skeletal maturation: what is normal? Radiology 250:28–38
Connolly S, Jaramillo D, Hong J et al (2004) Skeletal development in fetal pig specimens: MR imaging of femur with histologic comparison. Radiology 233:505–514
Wrathall A, Bailey J, Hebert C (1974) A radiographic study of development of the appendicular skeleton in the fetal pig. Res Vet Sci 17:154–168
Brighton C (1984) The growth plate. Orthop Clin North Am 15:571–595
Braden T (1993) Histophysiology of the growth plate and growth plate injuries. In: Smeak D, Bojrab J, Bloomberg M (eds) Disease mechanism in small animal surgery, 2nd edn. Lippincott Williams & Wilkins, Philadelphia, pp 1027–1041
Burdan F, Szumilo J, Korobowicz A et al (2009) Morphology and physiology of the epiphyseal growth plate. Folia Histochem Cytobiol 47:5–16
Turnbull HM (1930) Report upon the histology of the bone of the first case. J Pathol Bacteriol 33:332–338
Maxwell JP, Hu CH, Turnbull HM (1932) Fetal rickets. J Pathol Bacteriol 35:419–440
Muller H (1858) Ueber die Entwickelung der Knochensubstanz nebst Bemerkungen uber den Bau rachitischer Knochen. Zeitschr Wisscnsch Zool 9:147–233
Dodds GS (1932) Osteoclasts and cartilage removal in endochondral ossification of certain mammals. Am J Anat 50:97–127
Anderson H (1969) Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol 41:59–72
Park E (1964) The imprinting of nutritional disturbance on the growing bone. Pediatrics 33:815–862
Schenk R, Wiener J, Spiro D (1968) Fine structural aspects of vascular invasion of the tibial epiphyseal plate of growing rates. Acta Anat 69:1–17
Fazzalari NL, Moore AJ, Byers S et al (1997) Quantitative analysis of trabecular morphogenesis in the human costochondral junction during the postnatal period in normal subjects. Anat Rec 248:1–12
Steinbach HL, Noetzli M (1964) Roentgen appearance of the skeleton in osteomalacia and rickets. AJR Am J Roentgenol 91:955–972
Blickman JG, Wilkinson RH, Graef JW (1986) The radiologic “lead band” revisited. AJR Am J Roentgenol 146:245–247
Boeve WJ, Martijn A (1987) Case report 406: scurvy. Skeletal Radiol 16:67–69
Gerstenfeld L, Shapiro F (1996) Expression of bone-specific genes by hypertrophic chondrocytes: implication of the complex functions of the hypertrophic chondrocytes during endochondral bone development. J Cell Biochem 62:1–9
Ham AW, Cormack DH (1979) Histology, 8th edn. Lippincott Williams & Wilkins, Philadelphia
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsai, A., McDonald, A.G., Rosenberg, A.E. et al. Discordant radiologic and histological dimensions of the zone of provisional calcification in fetal piglets. Pediatr Radiol 43, 1606–1614 (2013). https://doi.org/10.1007/s00247-013-2740-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-013-2740-z