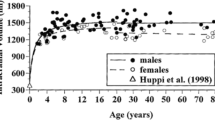
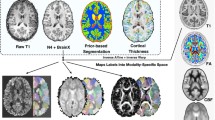
Abstract
The development of the human brain, from the fetal period until childhood, happens in a series of intertwined neurogenetical and histogenetical events that are influenced by environment. Neuronal proliferation and migration, cell aggregation, axonal ingrowth and outgrowth, dendritic arborisation, synaptic pruning and myelinisation contribute to the ‘plasticity of the developing brain’. These events taken together contribute to the establishment of adult-like neuroarchitecture required for normal brain function. With the advances in technology today, mostly due to the development of non-invasive neuroimaging tools, it is possible to analyze these structural events not only in anatomical space but also longitudinally in time. In this review we have highlighted current ‘state of the art’ neuroimaging tools. Development of the new MRI acquisition sequences (DTI, CHARMED and phase imaging) provides valuable insight into the changes of the microstructural environment of the cortex and white matter. Development of MRI imaging tools dedicated for analysis of the acquired images (i) TBSS and ROI fiber tractography, (ii) new tissue segmentation techniques and (iii) morphometric analysis of the cortical mantle (cortical thickness and convolutions) allows the researchers to map the longitudinal changes in the macrostructure of the developing brain that go hand-in-hand with the acquisition of cognitive skills during childhood. Finally, the latest and the newest technologies, like connectom analysis and resting state fMRI connectivity analysis, today, for the first time provide the opportunity to study the developing brain through the prism of maturation of the systems and networks beyond individual anatomical areas. Combining these methods in the future and modeling the hierarchical organization of the brain might ultimately help to understand the mechanisms underlying complex brain structure function relationships of normal development and of developmental disorders.




Similar content being viewed by others
References
Dubois J, Benders M, Borradori-Tolsa C et al (2008) Primary cortical folding in the human newborn: an early marker of later functional development. Brain 131:2028–2041
Dubois J, Benders M, Cachia A et al (2007) Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex 18:1444–1454
Dubois J, Benders M, Lazeyras F et al (2010) Structural asymmetries of perisylvian regions in the preterm newborn. Neuroimage 52:32–42
Barazany D, Assaf Y (2011) Visualization of cortical lamination patterns with magnetic resonance imaging. Cereb Cortex 22:2016–2023
Ducharme S, Hudziak JJ, Botteron KN et al (2012) Decreased regional cortical thickness and thinning rate are associated with inattention symptoms in healthy children. J Am Acad Child Adolesc Psychiatry 51:18–27
van Soelen I, Brouwer RM, van Baal GC et al (2012) Genetic influences on thinning of the cerebral cortex during development. Neuroimage 59:3871–3880
Dubois J, Haene-Lambertz G, Perrin M et al (2008) Asynchrony of the early maturation of white matter bundles in healthy infants: quantitative landmarks revealed noninvasively by diffusion tensor imaging. Hum Brain Mapp 29:14–27
Dubois J, Hertz-Pannier L, Dehaene-Lambertz G et al (2006) Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: a feasibility study using quantitative diffusion tensor imaging and tractography. Neuroimage 30:1121–1132
Hüppi P, Maier S, Peled S et al (1998) Microstructural development of human newborns cerebral white matter assessed in vivo by diffusion tensor MRI. Pediatr Res 44:584–590
Hüppi P, Murphy B, Maier S et al (2001) Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics 107:455–460
Bystron I, Blakemore C, Rakic P (2008) Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci 9:110–122
Rados M, Judas M, Kostovic I (2006) In vitro MRI of brain development. Eur J Radiol 57:187–198
Kostovic I, Vasung L (2009) Insights from in vitro fetal magnetic resonance imaging of cerebral development. Semin Perinatol 33:220–233
Lodygensky GA, Vasung L, Sizonenko SV et al (2010) Neuroimaging of cortical development and brain connectivity in human newborns and animal models. J Anat 217:418–428
Huang H, Xue R, Zhang J et al (2009) Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J Neurosci 29:4263–4273
McKinstry RC, Mathur A, Miller JH et al (2002) Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI2. Cereb Cortex 12:1237–1243
Neil J, Shiran S, McKinstry R et al (1998) Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR Imaging. Radiology 209:57–66
Vasung L, Huang H, Jovanov-Milosevic N et al (2010) Development of axonal pathways in the human fetal fronto-limbic brain: histochemical characterization and diffusion tensor imaging. J Anat 217:400–417
Kostovic I, Jovanov-Milosevic N (2006) The development of cerebral connections during the first 20–45 weeks’ gestation. Semin Fetal Neonatal Med 11:415–422
Giedd JN, Rapoport JL (2010) Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron 67:728–734
Anbeek P, Vincken KL, Groenendaal F et al (2008) Probabilistic brain tissue segmentation in neonatal magnetic resonance imaging. Pediatr Res 63:158–163
Cardoso MJ, Melbourne A, Kendall GS et al (2011) Adaptive neonate brain segmentation. Med Image Comput Comput Assist Interv 14:378–386
Gui L, Lisowski R, Faundez T et al (2012) Morphology-driven automatic segmentation of MR images of the neonatal brain. Med Image Anal. doi:10.1016/j.media.2012.07.006
Prastawa M, Gilmore JH, Lin W et al (2005) Automatic segmentation of MR images of the developing newborn brain. Med Image Anal 9:457–466
Weisenfeld NI, Warfield SK (2009) Automatic segmentation of newborn brain MRI. Neuroimage 47:564–572
Xue H, Srinivasan L, Jiang S et al (2007) Automatic cortical segmentation in the developing brain. Inf Process Med Imaging 20:257–269
Sowell ER, Peterson BS, Kan E et al (2007) Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex 17:1550–1560
Sowell ER, Thompson PM, Leonard CM et al (2004) Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci 24:8223–8231
Raznahan A, Lerch JP, Lee N et al (2011) Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron 72:873–884
Raznahan A, Shaw P, Lalonde F et al (2011) How does your cortex grow? J Neurosci 31:7174–7177
Shaw P, Greenstein D, Lerch J et al (2006) Intellectual ability and cortical development in children and adolescents. Nature 440:676–679
Kang HJ, Kawasawa YI, Cheng F et al (2012) Spatio-temporal transcriptome of the human brain. Nature 478:483–489
Lerch JP, Worsley K, Shaw WP et al (2006) Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage 31:993–1003
Clouchoux C, Kudelski D, Gholipour A et al (2012) Quantitative in vivo MRI measurement of cortical development in the fetus. Brain Struct Funct 217:127–139
Habas PA, Scott JA, Roosta A et al (2012) Early folding patterns and asymmetries of the normal human brain detected from in utero MRI. Cereb Cortex 22:13–25
Rajagopalan V, Scott J, Habas PA et al (2011) Spatiotemporal morphometry of adjacent tissue layers with application to the study of sulcal formation. Med Image Comput Comput Assist Interv 14:476–483
Lefevre J, Mangin JF (2010) A reaction-diffusion model of human brain development. PLoS Comput Biol 6:e1000749
Inder TE, Warfield SK, Wang H et al (2005) Abnormal cerebral structure is present at term in premature infants. Pediatrics 115:286–294
Biagioni E, Frisone MF, Laroche S et al (2007) Maturation of cerebral electrical activity and development of cortical folding in young very preterm infants. Clin Neurophysiol 118:53–59
Kapellou O, Counsell SJ, Kennea N et al (2006) Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med 3:e265
Kesler SR, Vohr B, Schneider KC et al (2005) Increased temporal lobe gyrification in preterm children. Neuropsychologia 44:445–453
Hasan A, McIntosh AM, Droese UA et al (2011) Prefrontal cortex gyrification index in twins: an MRI study. Eur Arch Psychiatry Clin Neurosci 261:459–465
Inder TE, Huppi PS, Zientara GP et al (1999) The postmigrational development of polymicrogyria documented by magnetic resonance imaging from 31 weeks’ postconceptional age. Ann Neurol 45:798–801
Scott JA, Habas PA, Rajagopalan V et al (2012) Volumetric and surface-based 3D MRI analyses of fetal isolated mild ventriculomegaly: brain morphometry in ventriculomegaly. Brain Struct Funct. doi:10.1007/s00429-012-0418-1
Rakic P (2004) Neuroscience. Genetic control of cortical convolutions. Science 303:1983–1984
Miyashita-Lin EM, Hevner R, Wassarman KM et al (1999) Early neocortical regionalization in the absence of thalamic innervation. Science 285:906–909
Kim MJ, Provenzale JM, Law M (2006) Magnetic resonance and diffusion tensor imaging in pediatric white matter diseases. Top Magn Reson Imaging 17:265–274
Song SK, Sun SW, Ramsbottom MJ et al (2002) Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17:1429–1436
Conturo TE, McKinstry RC, Aronovitz JA et al (1995) Diffusion MRI: precision, accuracy and flow effects. NMR Biomed 8:307–332
Hüppi PS (2011) Diffusion tensor MRI in brain development. In: Jones DK (ed) Diffusion MRI theory, methods and applications. Oxford University Press, New York, pp 500–517
Dubois J, Poupon C, Lethimonnier F et al (2006) Optimized diffusion gradient orientation schemes for corrupted clinical DTI data sets. MAGMA 19:134–143
Kim DH, Chung S, Vigneron DB et al (2008) Diffusion-weighted imaging of the fetal brain in vivo. Magn Reson Med 59:216–220
Takahashi E, Folkerth RD, Galaburda AM et al (2012) Emerging cerebral connectivity in the human fetal brain: an MR tractography study. Cereb Cortex 22:455–464
Ashburner J, Friston KJ (2001) Why voxel-based morphometry should be used. Neuroimage 14:1238–1243
Smith SM, Jenkinson M, Johansen-Berg H et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505
Hagmann P, Cammoun L, Gigandet X et al (2010) MR connectomics: principles and challenges. J Neurosci Methods 194:34–45
Hagmann P, Sporns O, Madan N et al (2010) White matter maturation reshapes structural connectivity in the late developing human brain. Proc Natl Acad Sci USA 107:19067–19072
Eluvathingal TJ, Hasan KM, Kramer L et al (2007) Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex 17:2760–2768
Anjari M, Srinivasan L, Allsop JM et al (2007) Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. Neuroimage 35:1021–1027
Giorgio A, Watkins KE, Douaud G et al (2008) Changes in white matter microstructure during adolescence. Neuroimage 39:52–61
Ball G, Counsell SJ, Anjari M et al (2010) An optimised tract-based spatial statistics protocol for neonates: applications to prematurity and chronic lung disease. Neuroimage 53:94–102
Bassi L, Chew A, Merchant N et al (2011) Diffusion tensor imaging in preterm infants with punctate white matter lesions. Pediatr Res 69:561–566
Porter EJ, Counsell SJ, Edwards AD et al (2010) Tract-based spatial statistics of magnetic resonance images to assess disease and treatment effects in perinatal asphyxial encephalopathy. Pediatr Res 68:205–209
Ment LR, Hirtz D, Huppi PS (2009) Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol 8:1042–1055
Qiu D, Tan LH, Zhou K et al (2008) Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage 41:223–232
Braun K (1996) Synaptic reorganization in early childhood experience and learning processes: relevance for the development of psychological diseases. Z Klin Psychol Psychiatr Psychother 44:253–266
Bengtsson SL, Nagy Z, Skare S et al (2005) Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci 8:1148–1150
Sagi Y, Tavor I, Hofstetter S et al (2012) Learning in the fast lane: new insights into neuroplasticity. Neuron 73:1195–1203
Assaf Y, Basser PJ (2005) Composite hindered and restricted model of diffusion (CHARMED) MR imaging of the human brain. Neuroimage 27:48–58
Assaf Y, Freidlin RZ, Rohde GK et al (2004) New modeling and experimental framework to characterize hindered and restricted water diffusion in brain white matter. Magn Reson Med 52:965–978
Assaf Y, Blumenfeld-Katzir T, Yovel Y et al (2008) AxCaliber: a method for measuring axon diameter distribution from diffusion MRI. Magn Reson Med 59:1347–1354
Alexander DC, Hubbard PL, Hall MG et al (2010) Orientationally invariant indices of axon diameter and density from diffusion MRI. Neuroimage 52:1374–1389
Duyn JH, van Gelderen P, Li TQ et al (2007) High-field MRI of brain cortical substructure based on signal phase. Proc Natl Acad Sci USA 104:11796–11801
Zhong K, Leupold J, von Elverfeldt ED et al (2008) The molecular basis for gray and white matter contrast in phase imaging. Neuroimage 40:1561–1566
Lodygensky GA, Marques JP, Maddage R et al (2012) In vivo assessment of myelination by phase imaging at high magnetic field. Neuroimage 59:1979–1987
Zhong K, Ernst T, Buchthal S et al (2011) Phase contrast imaging in neonates. Neuroimage 55:1068–1072
Yap PT, Fan Y, Chen Y et al (2011) Development trends of white matter connectivity in the first years of life. PLoS One 6:e24678
Batalle D, Eixarch E, Figueras F et al (2012) Altered small-world topology of structural brain networks in infants with intrauterine growth restriction and its association with later neurodevelopmental outcome. Neuroimage 60:1352–1366
Fransson P, Skiold B, Engstrom M et al (2009) Spontaneous brain activity in the newborn brain during natural sleep—an fMRI study in infants born at full term. Pediatr Res 66:301–305
Fransson P, Skiold B, Horsch S et al (2007) Resting-state networks in the infant brain. Proc Natl Acad Sci USA 104:15531–15536
Smyser CD, Inder TE, Shimony JS et al (2010) Longitudinal analysis of neural network development in preterm infants. Cereb Cortex 20:2852–2862
Smyser CD, Snyder AZ, Neil JJ (2011) Functional connectivity MRI in infants: exploration of the functional organization of the developing brain. Neuroimage 56:1437–1452
Gao W, Gilmore JH, Giovanello KS et al (2011) Temporal and spatial evolution of brain network topology during the first two years of life. PLoS One 6:e25278
Wedeen VJ, Rosene DL, Wang R et al (2012) The geometric structure of the brain fiber pathways. Science 335:1628–1634
Clouchoux C, Riviere D, Mangin JF et al (2010) Model-driven parameterization of the cortical surface for localization and inter-subject matching. Med Image Comput Comput Assist 50:552–566
Chen CH, Gutierrez ED, Thompson W et al (2012) Hierarchical genetic organization of human cortical surface area. Science 335:1634–1636
Zilles K, Amunts K (2012) Neuroscience. Segregation and wiring in the brain. Science 335:1582–1584
Acknowledgment
This work was supported by the Center for Biomedical Imaging (CIBM) of the Geneva and Lausanne Universities, the Ecole Polytechnique Fédérale de Lausanne (EPFL), the Geneva and Lausanne University Hospitals, as well as by the Leenards Foundation and the Swiss National Research Foundations to Professor Petra S. Hüppi, grant support FNS 32003B-113632, 33CM30-124101. We also thank Francois Lazeyras, Director of CIBM Geneva, for help with MRI acquisition of the newborns.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vasung, L., Fischi-Gomez, E. & Hüppi, P.S. Multimodality evaluation of the pediatric brain: DTI and its competitors. Pediatr Radiol 43, 60–68 (2013). https://doi.org/10.1007/s00247-012-2515-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-012-2515-y