Abstract
Background
We observed bone marrow signal changes (BMSC) in patients with plexiform neurofibromas after treatment with imatinib mesylate (Gleevec).
Objective
To evaluate the pattern and natural history of BMSC.
Materials and methods
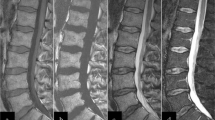
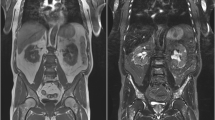
The data were obtained from a pilot study of imatinib mesylate in patients with plexiform neurofibromas. All patients underwent baseline and sequential whole-body STIR 1.5-T MRI after treatment. The bone marrow signal on MRI was evaluated for abnormalities, location and pattern, and any change on follow-up studies.
Results
The study group included 16 patients (8 males) with a median age of 14 years (range 4 to 25 years). The mean whole-body MRI follow-up duration was 1.9 years. Of the 16 patients, 14 (88%) developed BMSC. The signal change was asymmetrical in 9 of the 14 patients (64%). The appendicular skeleton was involved in all 14 patients and the axial skeleton in 3 patients (21%). BMSC was followed in 13 patients and decreased signal was seen in 9 patients (69%) after a mean duration of 1.3 years of treatment (range 0.6 to 2.9 years); no complications were observed.
Conclusion
BMSC appeared in most patients with neurofibromatosis type 1 following treatment with imatinib mesylate. BMSC was unusually asymmetrical and involved the lower extremities. On follow-up, BMSC often showed a decrease without complications.



Similar content being viewed by others
References
Borthakur G, Cortes JE (2004) Imatinib mesylate in the treatment of chronic myelogenous leukemia. Int J Hematol 79:411–419
Siddiqui MA, Scott LJ (2007) Imatinib: a review of its use in the management of gastrointestinal stromal tumours. Drugs 67:805–820
Jett K, Friedman JM (2010) Clinical and genetic aspects of neurofibromatosis 1. Genet Med 12:1–11
Zoller ME, Rembeck B, Oden A et al (1997) Malignant and benign tumors in patients with neurofibromatosis type 1 in a defined Swedish population. Cancer 79:2125–2131
Evans DG, Baser ME, McGaughran J et al (2002) Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet 39:311–314
Yang FC, Ingram DA, Chen S et al (2008) Nf1-dependent tumors require a microenvironment containing Nf1+/– and c-kit-dependent bone marrow. Cell 135:437–448
Demestre M, Herzberg J, Holtkamp N et al (2010) Imatinib mesylate (Glivec) inhibits Schwann cell viability and reduces the size of human plexiform neurofibroma in a xenograft model. J Neurooncol 98:11–19
McClelland CM, Harocopos GJ, Custer PL (2010) Periorbital edema secondary to imatinib mesylate. Clin Ophthalmol 4:427–431
Larson JS, Bergstrom LK, Cameron JD et al (2007) Severe periorbital edema secondary to imatinib mesylate for chronic myelogenous leukemia. Arch Ophthalmol 125:985–986
Esmaeli B, Prieto VG, Butler CE et al (2002) Severe periorbital edema secondary to STI571 (Gleevec). Cancer 95:881–887
Valeyrie L, Bastuji-Garin S, Revuz J et al (2003) Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukemias: a prospective study of 54 patients. J Am Acad Dermatol 48:201–206
Kelly K, Swords R, Mahalingam D et al (2009) Serosal inflammation (pleural and pericardial effusions) related to tyrosine kinase inhibitors. Target Oncol 4:99–105
Vanel D, Bonvalot S, Pechoux CL et al (2007) Imatinib-induced bone marrow necrosis detected on MRI examination and mimicking bone metastases. Skeletal Radiol 36:895–898
Tamura T, Tasaka T, Fujimoto M et al (2004) Massive bone marrow necrosis in a patient with chronic myelocytic leukemia following imatinib mesylate therapy. Haematologica 89:109–110
Berman E, Nicolaides M, Maki RG et al (2006) Altered bone and mineral metabolism in patients receiving imatinib mesylate. N Engl J Med 354:2006–2013
Pietras K, Ostman A, Sjoquist M et al (2001) Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res 61:2929–2934
Bankier AA, Levine D, Halpern EF et al (2010) Consensus interpretation in imaging research: is there a better way? Radiology 257:14–17
Conflicts of interest
We have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karmazyn, B., Cohen, M.D., Jennings, S.G. et al. Marrow signal changes observed in follow-up whole-body MRI studies in children and young adults with neurofibromatosis type 1 treated with imatinib mesylate (Gleevec) for plexiform neurofibromas. Pediatr Radiol 42, 1218–1222 (2012). https://doi.org/10.1007/s00247-012-2440-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-012-2440-0