Abstract
Background
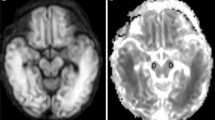
Brain edema during the early stages of hypoxic-ischemic/reperfusion (HI/R) injury can be determined using diffusion tensor imaging (DTI). The change in ADC values has been correlated with the change in expression of AQP-4.
Objective
To determine cerebral edema at specific time intervals after HI/R injury using DTI modalities and discuss its relationship to the expression of aquaporin-4 (AQP-4).
Materials and methods
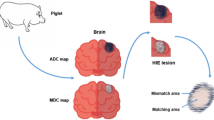
Thirty newborn piglets were divided into six groups (2, 6, 12, 24, 48 and 72 h) after HI/R injury. The control group subjected to sham surgery included five piglets. DTI scans and immunohistochemistry of AQP-4 expression were performed on piglet brain. The relationship between DTI parameters (FA and ADC values) and the optical density (OD) of AQP-4 expression was determined.
Results
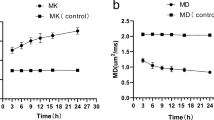
In the striatum, ADC values dropped and reached their lowest level at 24 h (F = 27.42, P < 0.05). In the subcortical border region, ADC values increased after a transient decrease and peaked at 48 h, demonstrating a significant difference from the control group (F = 50.25, P < 0.05). FA values in the internal capsules and subcortical white matter in HI/R models decreased continuously after HI/R, although no statistically significant difference from the control group was achieved. ADC and OD values of AQP-4 expression were positively correlated (r = 0.875, P < 0.05).
Conclusions
The change in ADC value after HI/R injury correlates with the expression of AQP-4.








Similar content being viewed by others
References
Vannucci RC (2000) Hypoxic-ischemic encephalopathy. Am J Perinatol 17:113–120
Whitelaw A, Thoresen M (2002) Clinical assessment and therapeutic interventions for hypoxic-ischemic encephalopathy in the full-term infant. Cambridge University Press, Cambridge
Volpe JJ (2008) Neurology of the newborn. W.B. Saunders Co., Philadelphia
Jin SH Jr, Edwards JN Jr (1997) Hypoxic-ischemic encephalopathy. In: Stevenson DK, Sunshine P (eds) Fetal and neonatal brain injury, 2nd edn. Oxford University Press, Oxford, pp 277–286
Perlman JM (2006) Summary proceedings from the neurology group on hypoxic-ischemic encephalopathy. Pediatrics 117:S28–S33
Lupton BA, Hill A, Roland EH et al (1988) Brain swelling in the asphyxiated term newborn: pathogenesis and outcome. Pediatrics 82:139–146
Meng S, Qiao M, Scobie K et al (2006) Evolution of magnetic resonance imaging changes associated with cerebral hypoxia-ischemia and a relatively selective white matter injury in neonatal rats. Pediatr Res 59:554–559
Qiao M, Latta P, Meng S et al (2004) Development of acute edema following cerebral hypoxia-ischemia in neonatal compared with juvenile rats using magnetic resonance imaging. Pediatr Res 55:101–106
Rutherford M, Counsell S, Allsop J et al (2004) Diffusion-weighted magnetic resonance imaging in term perinatal brain injury: a comparison with site of lesion and time from birth. Pediatrics 114:1004–1014
Winter JD, Lee DS, Hung RM et al (2007) Apparent diffusion coefficient pseudonormalization time in neonatal hypoxic-ischemic encephalopathy. Pediatr Neurol 37:255–262
McKinstry RC, Miller JH, Snyder AZ et al (2002) A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology 24:824–833
Ward P, Counsell S, Allsop J et al (2006) Reduced fractional anisotropy on diffusion tensor magnetic resonance imaging after hypoxic-ischemic encephalopathy. Pediatrics 117:e619–e630
Schmitz T, Ritter J, Mueller S et al (2011) Cellular changes underlying hyperoxia-induced delay of white matter development. J Neurosci 31:4327–4344
Righini A, Doneda C, Parazzini C et al (2010) Diffusion tensor imaging of early changes in corpus callosum after acute cerebral hemisphere lesions in newborns. Neuroradiology 52:1025–1035
Meng S, Qiao M, Lin L et al (2004) Correspondence of AQP4 expression and hypoxic-ischaemic brain oedema monitored by magnetic resonance imaging in the immature and juvenile rat. Eur J Neurosci 19:2261–2269
Badaut J, Hirt L, Granziera C et al (2001) Astrocyte-specific expression of aquaporin-9 in mouse brain is increased after transient focal cerebral ischemia. J Cereb Blood Flow Metab 21:477–482
de Castro RM, Hirt L, Bogousslavsky J et al (2006) Time course of aquaporin expression after transient focal cerebral ischemia in mice. J Neurosci Res 83:1231–1240
Badaut J, Lasbennes F, Magistretti PJ et al (2002) Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab 22:367–378
Manley GT, Fujimura M, Ma T et al (2000) Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 6:159–163
Papadopoulos MC, Manley GT, Krishna S et al (2004) Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J 18:1291–1293
Zhao J, Moore AN, Clifton GL et al (2005) Sulforaphane enhances aquaporin 4 expression and decreases cerebral edema following traumatic brain injury. J Neurosci Res 82:499–506
Pond WG, Boleman SL, Fiorotto ML et al (2000) Perinatal ontogeny of brain growth in the domestic pig. Proc Soc Exp Biol Med 223:102–108
McLean C, Ferriero D (2004) Mechanisms of hypoxic-ischemic injury in the term infant. Elsevier, Amsterdam, pp 425–432
Fu X, Li Q, Feng Z et al (2007) The roles of aquaporin 4 in brain edema following neonatal hypoxia ischemia and reoxygenation in a cultured rat astrocyte model. Glia 55:935–941
Klatzo I (1994) Evolution of brain edema concepts. Acta Neurochir Suppl (Wien) 60:3–6
Loubinoux I, Volk A, Borredon J et al (1997) Spreading of vasogenic edema and cytotoxic edema assessed by quantitative diffusion and T2 magnetic resonance imaging. Stroke 28:419–427
Nedelcu J, Klein MA, Aguzzi A et al (1999) Biphasic edema after hypoxic-ischemic brain injury in neonatal rats reflects early neuronal and late glial damage. Pediatr Res 46:297–304
Lu H, Sun SQ (2003) A correlative study between AQP4 expression and the manifestation of DWI after the acute ischemic brain edema in rats. Chin Med J (Engl) 116:1063–1069
Rutherford M, Pennock J, Schwieso J et al (1996) Hypoxic-ischaemic encephalopathy: early and late magnetic resonance imaging findings in relation to outcome. Arch Dis Child Fetal Neonatal Ed 75:F145–F151
Rutherford MA, Pennock JM, Counsell SJ et al (1998) Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics 102:323–328
Munkeby BH, De Lange C, Emblem KE et al (2008) A piglet model for detection of hypoxic-ischemic brain injury with magnetic resonance imaging. Acta Radiol 49:1049–1057
Brissaud O, Amirault M, Villega F et al (2010) Efficiency of fractional anisotropy and apparent diffusion coefficient on diffusion tensor imaging in prognosis of neonates with hypoxic-ischemic encephalopathy: a methodologic prospective pilot study. AJNR 31:282–287
Forsyth R (1996) Astrocytes and the delivery of glucose from plasma to neurons. Neurochem Int 28:231–241
Kirchhoff F, Dringen R, Giaume C (2001) Pathways of neuron-astrocyte interactions and their possible role in neuroprotection. Eur Arch Psychiatry Clin Neurosci 251:159–169
Nakata N, Kato H, Kogure K (1993) Protective effects of basic fibroblast growth factor against hippocampal neuronal damage following cerebral ischemia in the gerbil. Brain Res 605:354–356
Song H, Stevens CF, Gage FH (2002) Astroglia induce neurogenesis from adult neural stem cells. Nature 417:39–44
Haupt C, Witte O, Frahm C (2007) Temporal profile of connexin 43 expression after photothrombotic lesion in rat brain. Neuroscience 144:562–570
Wang S, Wu EX, Cai K et al (2009) Mild hypoxic-ischemic injury in the neonatal rat brain: longitudinal evaluation of white matter using diffusion tensor MR imaging. AJNR 30:1907–1913
Acknowledgments
This work was supported by the State Natural Sciences Foundation of China (No. 30570541). We appreciate the valuable comments from other members of our laboratories.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Wang, X. & Guo, Q. The correlation between DTI parameters and levels of AQP-4 in the early phases of cerebral edema after hypoxic-ischemic/reperfusion injury in piglets. Pediatr Radiol 42, 992–999 (2012). https://doi.org/10.1007/s00247-012-2373-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-012-2373-7