Abstract
Background
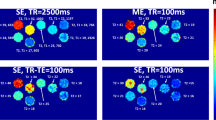
MRI is being increasingly used to evaluate tissue relaxation in the setting of iron overload. Diagnostic accuracy is strongly dependent upon the acquisition and analysis methods employed. Typically, a multi-echo train of relaxation data is acquired, the resulting curve is fit using a non-linear (exponential) function, and the derived relaxation time is converted to iron concentration by a calibration formula derived from paired MRI-biopsy samples. A theoretically valid processing alternative is to fit a straight line to the relaxation data after logarithmic transformation (log-linear). This log-linear method is more computationally efficient, allowing a full relaxation map to be generated in near real time. This method is present on all scanner platforms and has been published for use in assessing iron concentration. These factors imply methodological validity.
Objective
To use in vivo and simulation data to show that log-linear fitting can generate highly erroneous relaxation results in iron-loaded tissues.
Materials and methods
After IRB approval, exponential and linear fitting were compared in a cohort of 20 patients being evaluated for hepatic iron overload. Simulation analyses were performed to characterize the main factors impacting derived results.
Results
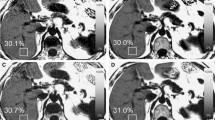
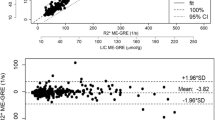
In human subjects, log-linear analyses demonstrated gross deviation from exponential results at a moderate relaxation shortening (T2* ~5 ms). Simulation analyses demonstrated that the discrepancy was caused by noise effects and additional signal components violating mono-exponential function shape.
Conclusion
Log-linear processing results in increasingly erroneous estimation of T2* with iron-loading. Therefore, this method should not be employed for measurement of relaxation behavior in clinical samples.





Similar content being viewed by others
References
Bulte JW, Miller GF, Vymazal J et al (1997) Hepatic hemosiderosis in non-human primates: quantification of liver iron using different field strengths. Magn Reson Med 37:530–536
Anderson LJ, Holden S, Davis B et al (2001) Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J 22:2171–2179
Clark PR, St Pierre TG (2000) Quantitative mapping of transverse relaxivity (1/T(2)) in hepatic iron overload: a single spin-echo imaging methodology. Magn Reson Imaging 18:431–438
Hankins JS, McCarville MB, Loeffler RB et al (2009) R2* magnetic resonance imaging of the liver in patients with iron overload. Blood 113:4853–4855
St. Pierre TG, Clark PR, Chua-Anusorn W et al (2005) Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 105:855–861
Stark DD, Moseley ME, Bacon BR et al (1985) Magnetic resonance imaging and spectroscopy of hepatic iron overload. Radiology 154:137–142
Wood JC, Enriquez C, Ghugre N et al (2005) MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 106:1460–1465
Olivieri NF, Brittenham GM (1997) Iron-chelating therapy and the treatment of thalassemia. Blood 89:739–761
Meloni A, Ramazzotti A, Positano V et al (2009) Evaluation of a web-based network for reproducible T2* MRI assessment of iron overload in thalassemia. Int J Med Inform 78:503–512
Positano V, Salani B, Pepe A et al (2009) Improved T2* assessment in liver iron overload by magnetic resonance imaging. Magn Reson Imaging 27:188–197
Song R, Cohen AR, Song HK (2007) Improved transverse relaxation rate measurement techniques for the assessment of hepatic and myocardial iron content. J Magn Reson Imaging 26:208–214
Voskaridou E, Douskou M, Terpos E et al (2004) Magnetic resonance imaging in the evaluation of iron overload in patients with beta thalassaemia and sickle cell disease. Br J Haematol 126:736–742
Argyropoulou MI, Astrakas L (2007) MRI evaluation of tissue iron burden in patients with beta-thalassaemia major. Pediatr Radiol 37:1191–1200, quiz 1308–1309
Leone FC, Nelson LS, Nottingham RB (1961) The folded normal distribution. Technometrics 3:543–550
Cardenas-Blanco A, Tejos C, Irarrazaval P et al (2008) Noise in magnitude magnetic resonance images. Concepts Magn Reson 32:409–416
Jensen JH, Tang H, Tosti CL et al (2010) Separate MRI quantification of dispersed (ferritin-like) and aggregated (hemosiderin-like) storage iron. Magn Reson Med 63:1201–1209
Jensen JH, Chandra R (2000) Strong field behavior of the NMR signal from magnetically heterogeneous tissues. Magn Reson Med 43:226–236
Jensen JH, Chandra R (2002) Theory of nonexponential NMR signal decay in liver with iron overload or superparamagnetic iron oxide particles. Magn Reson Med 47:1131–1138
Ghugre NR, Coates TD, Nelson MD et al (2005) Mechanisms of tissue-iron relaxivity: nuclear magnetic resonance studies of human liver biopsy specimens. Magn Reson Med 54:1185–1193
Raya JG, Dietrich O, Horng A et al (2010) T2 measurement in articular cartilage: impact of the fitting method on accuracy and precision at low SNR. Magn Reson Med 63:181–193
Acknowledgement
This work was presented at the IPR 2011 in London.
We acknowledge the helpful comments of Dr. David Purdy at Siemens Medical Systems regarding noise distributions and line-fitting effects.
This work was supported by grant R01 AG030484
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Otto, R., Ferguson, M.R., Marro, K. et al. Limitations of using logarithmic transformation and linear fitting to estimate relaxation rates in iron-loaded liver. Pediatr Radiol 41, 1259–1265 (2011). https://doi.org/10.1007/s00247-011-2082-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-011-2082-7