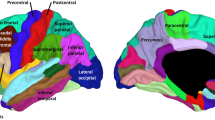
Abstract
Background
Determination of diffusion tensor metrics in typically developing school-age children shows that maturational increases in fractional anisotropy (FA) vary across the brain and that age effects on FA are to increases in axial diffusivity in some regions, to decreases in radial diffusivity in some, and to both increases in axial and decreases in radial diffusivity in others.
Objective
When studying developing white matter (WM) using diffusion tensor imaging (DTI), knowledge of age-related normative tensor metrics is important, as normal variations can mask or mimic disease effects.
Materials and methods
Right-handed English-speaking children (n = 32) 6–18 years old (mean 11.0) were studied over 31 months, 7 longitudinally. Anisotropy data were analyzed using tract-based spatial statistics; 43 regions showing significant (P < 0.05) age effects on fractional anisotropy (FA) were analyzed for age effects (r), coefficient of variability (CV), and FA, axial and radial diffusivity. This study was IRB-approved.
Results
The callosal genu and splenium showed the highest FA values, smallest age effects, and lowest between-subject variability. Mean FA was lower and age effects were greatest in the dorsal callosal body. The highest age effects on FA were in the cingulum, centrum semiovale, right corticospinal tract, and right temporal WM. The dorsal callosal body, calcarine WM, superior frontal and temporal gyri, and right corticospinal tract showed the highest CV. Radial diffusivity decreased while axial diffusivity increased in the cingulum, decreased in the optic tracts, and showed minimal or no age effects in most other regions.
Conclusion
Age effects on FA and variability in FA are location-dependant in developing WM.







Similar content being viewed by others
References
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111:209–219
Le Bihan D, Mangin JF, Poupon C et al (2001) Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13:534–546
Basser PJ, Jones DK (2002) Diffusion-tensor MRI: theory, experimental design and data analysis — a technical review. NMR Biomed 15:456–467
Jespersen SN, Kroenke CD, Ostergaard L et al (2007) Modeling dendrite density from magnetic resonance diffusion measurements. Neuroimage 34:1473–1486
Song SK, Yoshino J, Le TQ et al (2005) Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26:132–140
Harsan LA, Poulet P, Guignard B et al (2006) Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res 83:392–402
Hasan KM (2006) Diffusion tensor eigenvalues or both mean diffusivity and fractional anisotropy are required in quantitative clinical diffusion tensor MR reports: fractional anisotropy alone is not sufficient. Radiology 239:611–613
Hoeft F, Barnea-Goraly N, Haas BW et al (2007) More is not always better: increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. J Neurosci 27:11960–11965
Silk TJ, Vance A, Rinehart N et al (2009) White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum Brian Mapp 30:2757–2765
Hamilton LS, Levitt JG, O’Neill J et al (2008) Reduced white matter integrity in attention-deficit hyperactivity disorder. NeuroReport 19:1705–1708
Skranes J, Vangberg TR, Kulseng S et al (2007) Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain 130:654–666
Constable RT, Ment LR, Vohr BR et al (2008) Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics 121:306–316
Khong PL, Leung LH, Chan GC et al (2005) White matter anisotropy in childhood medulloblastoma survivors: association with neurotoxicity risk factors. Radiology 236:647–652
Alexander AL, Lee JE, Lazar M et al (2007) Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage 34:61–73
Yuan W, Holland SK, Schmithorst VJ et al (2007) Diffusion tensor MR imaging reveals persistent white matter alteration after traumatic brain injury experienced during early childhood. AJNR 28:1919–1925
Richards T, Stevenson J, Crouch LC et al (2008) Tract-based spatial statistics of diffusion tensor imaging in adults with dyslexia. AJNR 29:1134–1139
Deutsch GK, Dougherty RF, Bammer R et al (2005) Children’s reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex 41:354–363
Jones DK, Horsfield MA, Simmons A (1999) Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med 42:515–525
Friedman L, Glover GH (2006) Report on a multicenter fMRI Quality Assurance Protocol. J Magn Reson Imaging 23:827–839
Netsch T, van Muiswinkel A (2004) Quantitative evaluation of image-based distortion correction in diffusion tensor imaging. IEEE Trans Med Imaging 23:789–798
Smith SM, Jenkinson M, Johansen-Berg H et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505
Hofer S, Frahm J (2006) Topography of the human corpus callosum revisited — comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 32:989–994
Mori S, Wakana S, Nagae-Poetscher LM et al (2005) MRI atlas of human white matter. Elsevier BV, Amsterdam
Snedecor GW, Cochran WG (1980) Statistical methods, 7th edn. Iowa State University Press, Ames
Barnea-Goraly N, Menon V, Eckert M et al (2005) White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex 15:1848–1854
Eluvathingal TJ, Hasan KM, Kramer L et al (2007) Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex 17:2760–2768
Gao W, Lin W, Chen Y et al (2009) Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. AJNR 30:290–296
Giorgio A, Watkins KE, Chadwick M et al (2010) Longitudinal changes in grey and white matter during adolescence. Neuroimage 49:94–103
Hermoye L, Saint-Martin C, Cosnard G et al (2006) Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage 29:493–504
Ashtari M, Cervellione KL, Hasan KM et al (2007) White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. Neuroimage 35:501–510
Qiu D, Tan LH, Zhou K et al (2008) Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage 41:223–232
Basser PJ, Pajevic S, Pierpaoli C et al (2000) In vivo fiber tractography using DT-MRI data. Magn Reson Med 44:625–632
Mori S, Kaufmann WE, Davatzikos C et al (2002) Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med 47:215–223
Mukherjee P, Chung SW, Berman JI et al (2008) Diffusion tensor MR imaging and fiber tractography: technical considerations. AJNR 29:843–852
Snook L, Plewes C, Beaulieu C (2007) Voxel based versus region of interest analysis in diffusion tensor imaging of neurodevelopment. Neuroimage 34:243–252
Jones DK, Symms MR, Cercignani M et al (2005) The effect of filter size on VBM analyses of DT-MRI data. Neuroimage 26:546–554
Rollins NK, Morris MC, Chia JM et al (2010) Comparison of FA values from TBSS vs. manual ROI analysis. Presented at the 19th Annual Meeting of the International Society of Magnetic Resonance in Medicine. Stockholm Sweden, May 2010
Takei K, Yamasue H, Abe O et al (2009) Structural disruption of the dorsal cingulum bundle is associated with impaired Stroop performance in patients with schizophrenia. Schizophr Res 114:119–127
Wang F, Jackowski M, Kalmar JH et al (2008) Abnormal anterior cingulum integrity in bipolar disorder determined through diffusion tensor imaging. Br J Psychiatry 193:126–129
Ozturk A, Sasson AD, Farrell JA et al (2008) Regional differences in diffusion tensor imaging measurements: assessment of intrarater and interrater variability. AJNR 29:1124–1127
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rollins, N.K., Glasier, P., Seo, Y. et al. Age-related variations in white matter anisotropy in school-age children. Pediatr Radiol 40, 1918–1930 (2010). https://doi.org/10.1007/s00247-010-1744-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-010-1744-1