Abstract
As the number of patients with liver transplants continues to increase, radiologists need to be aware of the normal post-operative appearance of the different liver transplants currently performed along with the wide variety of complications encountered. The complications commonly affect the biliar and vascular systems and can include anastomotic bile leakage and biliary stenosis along with stenosis or obstruction of the hepatic artery, portal or hepatic veins and IVC. Other complications include parenchymal abnormalities such as hepatic infarction, organ rejection, localized collections and post transplant lymphoproliferative disorder. This article reviews and illustrates the role of imaging for pediatric transplantation including the role of interventional radiology.
Similar content being viewed by others
Liver transplantation
Transplantation has become an established treatment for many hepatic conditions and indeed is often the last resort for patient survival. The number of transplantations is increasing as improved surgical techniques, immunosuppression, and postoperative care have increased success rates [1, 2]. The common types of liver transplant are discussed in Table 1. With the increasing number of liver transplantations have come new challenges for the radiologist in terms of pre- and posttransplant imaging. Preoperative imaging is often done to evaluate the potential donor to exclude any significant pathology and to obtain anatomical information for operative planning. Following transplantation imaging is mainly performed to evaluate complications, which may be acute or chronic [3].
The principles of pre- and posttransplant imaging of the liver in children are outlined here along with posttransplantation lymphoproliferative disorder (PTLD). Rejection of the transplanted organ or tissue occurs when the recipient’s immune system attacks it and causes damage to the organ; this can have systemic effects. Although transplant rejection is one of the more common complications, it does not have specific radiological features [4].
Pretransplant imaging
Pretransplant imaging is often performed to evaluate the donor as well as recipient [5]. Live donor organ imaging may be performed to evaluate hepatic size and vasculature ensuring suitability for transplant. Length, caliber and anatomic variations of both vessels and the biliary system are important for surgical planning. The usual modalities used include US, CT and MR.
Common indications for liver transplantation in children include biliary diseases, such as biliary atresia, sclerosing cholangitis, cystic fibrosis, primary biliary cirrhosis; along with metabolic diseases, such as alpha-1 antitrypsin deficiency, glycogen storage disease, Wilson disease, hemochromatosis; and cirrhosis from any cause.
Presently used grafts include pediatric cadaveric whole-organ graft, segmental or split adult cadaveric grafts, and living related adult segmental graft (segments II and III or II–IV). Use of split cadaveric graft and living donor segmental graft has increased the donor pool. Usually the donor hepatic artery, portal vein, suprahepatic IVC and infraheaptic IVC are anastomosed end-to-end to the recipient respective vessels. If the vascular pedicle is short, especially in split and segmental grafts, autologous iliac artery conduit or donor conduit from the infrarenal aorta can be used. However, this increases the risk of vascular complications. When recipient vena cava is kept as it is and donor hepatic vein is attached end-to-side to it, the technique is called ‘piggyback’ technique. Biliary anastomosis is typically performed in an end-to-end fashion except in biliary atresia where hepatojejunostomy is performed.
Posttransplant imaging
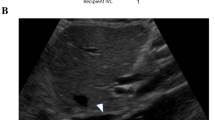
Vascular complications usually occur in the early postoperative period (Table 2). As the hepatic artery is the sole supply to the biliary epithelium of transplanted liver its patency is vital for graft survival. Caliber difference between donor and recipient vessels may be normally seen. Hemodynamically significant stenosis is diagnosed when a three-to fourfold increase in velocity is seen at the site of narrowing (Figs. 1, 2 and 3). Intrahepatic parvus tardus pattern may be normally seen in the first 72 h after transplant because of edema at the anastomotic site; hence, this finding should be interpreted with caution.
a Portal vein in a child with segmental liver transplant shows caliber difference in recipient (arrows) and donor (arrowheads) portal veins. b Percutaneous portal venogram in another child performed 6 years after liver transplant for biliary atresia shows stricture of the portal vein at the porta (arrow)
Biliary complications are the most common complications seen after pediatric liver transplant (in up to 27% of cases) and the majority of them occur in first 3 months following surgery (Fig. 4) [6–9]. They include anastomotic leakage and stenosis with proximal dilatation, bile duct stones, sludge, bilioma and rare mucocele of cystic duct remnant. Bile duct leak or stricture can lead to cholangitis, sepsis and abscess. Nonanastomotic strictures are probably related to hepatic arterial insufficiency. Posttransplantation biliary leak should prompt a search for hepatic artery thrombosis [21].
Bile leak with biloma in a 2 month-old-baby with segmental liver transplant for biliary atresia. Ultrasound showed marked dilatation of intrahepatic bile duct and a complex collection on inferior aspect of the liver (a). Percutaneous transhepatic cholangiogram confirmed the bile leak communicating with the collection compatible with bilioma (b)
Liver parenchymal abnormalities such as infarction, biliomas or abscess can complicate transplantation. Infarction can be seen as a round or geographical hypoechoic solid lesion on ultrasound. It is seen as an irregular, peripherally located wedge-shaped hypoattenuating lesion on CT images. A periportal area of low echogenicity on US and hypodensity on CT, called ‘periportal collar sign’, can be seen normally and is thought to result from dilatation of lymphatic channels. It usually resolves within few weeks. Organ rejection is a common complication; however, it does not have specific imaging features.
Other complications include localized collections, extrahepatic biliomas and post transplant lymphoproliferative disorder (PTLD).
Posttransplantation lymphoproliferative disorder
PTLD is a spectrum of unregulated lymphoid expansion that can occur in the transplant patients and range from polyclonal hyperplasia to monoclonal malignant lymphoma [10, 22–27]. It is related to chronic immunosuppression and in most cases, results from Epstein-Barr virus (EBV)-induced B cell lymphoproliferation. Overall the frequency of PTLD is around 2% but is seen with higher frequency (approximately 8%) in children. Three major risk factors include allograft type, EBV infection or reactivation and intense immunosuppressive regimens. About 85% of PTLD cases are of B cell origin and contain EBV. Most of the PTLD is seen in the first year after transplantation and presents with variable clinical manifestations. PTLD can involve any organ system including the allograft. In descending order of frequency it involves the abdomen, chest, head and neck, and brain. Histologically, three forms are seen: hyperplastic (early lesion), polymorphic and monomorphic (lymphomas). Polymorphic form has better prognosis and is more likely to respond to reduction in immunosuppressive therapy.
CT is the main modality for evaluation of PTLD in terms of presence, extent and biopsy guidance. Hypodense nodular masses or diffuse infiltration and enlargement can be seen in the liver, spleen and kidneys. Circumferential wall thickening, dilatation, ulceration and intussusception can be seen in the bowel with small bowel being most frequently involved. Abdominal lymphadenopathy, omental and mesenteric involvement are other manifestations. PTLD in the chest can manifest as discrete nodular masses, air-space consolidation that do not respond to antibiotics therapy and mediastenal lymphadenopathy. Diffuse enlargement of the pharyngeal and palatine tonsils and cervical lymphadenopathy is seen in head and neck PTLD. Sinonasal involvement by PTLD cannot be distinguished from an infective process radiologically. Solitary brain lesion is the most frequent manifestation of brain PTLD [28].
The radiologist plays a key role in evaluation of acute and chronic complications of pediatric liver transplantation. As these patients live longer, we must be vigilant in assessing for other long-term complications including pulmonary hypertension [29].
References
Otte JB (2002) History of pediatric liver transplantation. Where are we coming from? Where do we stand? Pediatr Transplant 6(5):378–387
Ryckman FC, Bucuvalas JC et al (2008) Outcomes following liver transplantation. Semin Pediatr Surg 17(2):123–130
Westra SJ, Zaninovic AC et al (1993) Imaging in pediatric liver transplantation. Radiographics 13(5):1081–1099
Ametani F, Itoh K et al (2001) Spectrum of CT findings in pediatric patients after partial liver transplantation. Radiographics 21(1):53–63
Sahani D, D’Souza R et al (2004) Evaluation of living liver transplant donors: method for precise anatomic definition by using a dedicated contrast-enhanced MR imaging protocol. Radiographics 24(4):957–967
Chan SC, Fan ST (2008) Biliary complications in liver transplantation. Hepatol Int 2(4):399–404
Fulcher AS, Turner MA (1999) Orthotopic liver transplantation: evaluation with MR cholangiography. Radiology 211(3):715–722
Sheng R, Ramirez CB et al (1996) Biliary stones and sludge in liver transplant patients: a 13-year experience. Radiology 198(1):243–247
Uribe M, Hunter B et al (2009) Posttransplant lymphoproliferative disorder in pediatric liver transplantation. Transplant Proc 41(6):2679–2681
Caiado AH, Blasbalg R et al (2007) Complications of liver transplantation: multimodality imaging approach. Radiographics 27(5):1401–1417
Crossin JD, Muradali D et al (2003) US of liver transplants: normal and abnormal. Radiographics 23(5):1093–1114
Garcia-Criado A, Gilabert R et al (2009) Doppler ultrasound findings in the hepatic artery shortly after liver transplantation. AJR 193(1):128–135
Jones VS, McCall JL et al (2009) Reverse portal flow after liver transplantation-ominous or acceptable? Pediatr Transplant. doi:10.1111/j.1399-3046.2008.01121.x
Marder DM, DeMarino GB et al (1989) Liver transplant rejection: value of the resistive index in Doppler US of hepatic arteries. Radiology 173(1):127–129
Nolten A, Sproat IA (1996) Hepatic artery thrombosis after liver transplantation: temporal accuracy of diagnosis with duplex US and the syndrome of impending thrombosis. Radiology 198(2):553–559
Pandharipande PV, Lee VS et al (2001) Vascular and extravascular complications of liver transplantation: comprehensive evaluation with three-dimensional contrast-enhanced volumetric MR imaging and MR cholangiopancreatography. AJR 177(5):1101–1107
Richard HM 3rd, Silberzweig JE et al (2000) Hepatic arterial complications in liver transplant recipients treated with pretransplantation chemoembolization for hepatocellular carcinoma. Radiology 214(3):775–779
Rossi AR, Pozniak MA et al (1993) Upper inferior vena caval anastomotic stenosis in liver transplant recipients: Doppler US diagnosis. Radiology 187(2):387–389
Ueda M, Oike F et al (2008) Portal vein complications in pediatric living donor liver transplantation using left-side grafts. Am J Transplant 8(10):2097–2105
Zajko AB, Bron KM et al (1985) Angiography of liver transplantation patients. Radiology 157(2):305–311
Berrocal T, Parron M et al (2006) Pediatric liver transplantation: a pictorial essay of early and late complications. Radiographics 26(4):1187–1209
Borhani AA, Hosseinzadeh K et al (2009) Imaging of posttransplantation lymphoproliferative disorder after solid organ transplantation. Radiographics 29(4):981–1000, discussion 1000–1002
Dhillon MS, Rai JK et al (2007) Post-transplant lymphoproliferative disease in liver transplantation. Br J Radiol 80:337–346
Donnelly LF, Frush DP et al (1998) Lymphoproliferative disorders: CT findings in immunocompromised children. AJR 171(3):725–731
McCormack L, Hany TI et al (2006) How useful is PET/CT imaging in the management of post-transplant lymphoproliferative disease after liver transplantation? Am J Transplant 6(7):1731–1736
Scarsbrook AF, Warakaulle DR et al (2005) Post-transplantation lymphoproliferative disorder: the spectrum of imaging appearances. Clin Radiol 60(1):47–55
Wu L, Rappaport DC et al (2001) Lymphoproliferative disorders after liver transplantation: imaging features. Abdom Imaging 26(2):200–206
Bianchi E, Pascual M et al (2008) Clinical usefulness of FDG-PET/CT scan imaging in the management of posttransplant lymphoproliferative disease. Transplantation 85(5):707–712
Koch DG, Caplan M et al (2009) Pulmonary hypertension after liver transplantation: case presentation and review of the literature. Liver Transpl 15(4):407–412
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. Paul S. Babyn has indicated that he has no relevant financial relationships or potential conflicts of interest related to the material presented.
Rights and permissions
About this article
Cite this article
Babyn, P.S. Imaging of the transplant liver. Pediatr Radiol 40, 442–446 (2010). https://doi.org/10.1007/s00247-010-1545-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-010-1545-6