Abstract
X-ray dose reduction in pediatrics is particularly important because babies and children are very sensitive to radiation exposure. We present new developments to further decrease pediatric patient dose. With the help of an advanced exposure control, a constant image quality can be maintained for all patient sizes, leading to dose savings for babies and children of up to 30%. Because objects of interest are quite small and the speed of motion is high in pediatric patients, short pulse widths down to 4 ms are important to reduce motion blurring artifacts. Further, a new noise-reduction algorithm is presented that detects and processes signal and noise in different frequency bands, generating smooth images without contrast loss. Finally, we introduce a super-resolution technique: two or more medical images, which are shifted against each other in a subpixel region, are combined to resolve structures smaller than the size of a single pixel. Advanced exposure control, short exposure times, noise reduction and super-resolution provide improved image quality, which can also be invested to save radiation exposure. All in all, the tools presented here offer a large potential to minimize the deterministic and stochastic risks of radiation exposure.
Similar content being viewed by others
Introduction
Because newborns and children are very sensitive to radiation exposure, X-ray imaging systems and equipment should be designed to reduce radiation dose for small patients. The following state-of-the-art techniques are well established: automatic exposure control, automatic prefilter exchange, adjustable pulse frequency down to 0.5 frames per second, radiation-free adjustment of the primary and semitransparent collimators, object positioning without radiation, measurement and display of the active area dose product and the accumulated skin dose, removable grids, and the option to store fluoro images.
Nevertheless, there is still potential for further dose reductions. Advanced exposure controls can further reduce patient radiation dose by varying detector doses. Short pulse widths prevent motion blurring for small and fast-moving objects. New algorithms can distinguish between noise and signal, enabling them to amplify the signal and reduce the noise. Finally, a super-resolution technique can improve the image resolution beyond the pixel resolution.
Materials and methods
Advanced exposure control
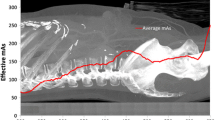
Image quality is mainly dependent on the contrast and the noise. Whereas the noise is determined by the detector dose, the contrast is basically related to the X-ray spectrum used. Hence, common X-ray systems maintain a constant tube voltage and detector dose for pediatric patients of all sizes. There are, however, further influences on the image quality. Newborns and children do not harden the X-ray spectrum as much as adults do, i.e., the loss of contrast due to patient beam hardening is lower. Furthermore, scatter radiation is highly dependent on patient thickness; the amount of scatter radiation in the case of newborns and children is smaller, hence, contrast loss due to scatter radiation is less expressed. A newly developed exposure control automatically handles these influences by optimizing the spectrum (tube voltage and prefiltering) and the detector dose for every patient thickness and for every requested image quality to achieve the lowest possible patient dose. The optimization is based on the calculations described by Bernhardt et al. [1].
Short pulse widths
High-speed motion caused by the heart beating, breathing, or patient motion causes blurring, which is particularly harmful for image quality if small objects are moving quickly, as in pediatric patients. As the noise level in fluoro is quite high, motion blurring cannot be eliminated by edge enhancement via software. Short pulse widths are, therefore, the only method to minimize motion blurring artifacts for fluoro and acquisition.
Noise-reduction algorithm
Noise reduction is one of the most challenging problems in fluoroscopy. Intelligent image processing is more and more able to distinguish between signal and noise, which is also a result of growing computational capabilities. Hence, the signal can be amplified, whereas the amount of noise can be reduced. New algorithms are based on Laplacian or Gaussian pyramids, splitting the image into different frequency bands. In each band the signal and the noise are identified and treated individually. Finally all the processed bands are combined to achieve an artifact-free, low-noise image. This kind of image processing can be performed at a frequency up to 30 frames per second.
Super-resolution technique
In pediatric patients, image resolution is of notable importance. Resolution is mainly dependent on the focal spot size and the pixel size of the detector. Resolution can be improved by the super-resolution technique. Here, two or more medical images that are translated, rotated and scaled against each other within at least the subpixel dimension are combined to resolve structures smaller than a single-detector pixel. These high-resolution images can help to reduce the total radiation time during diagnosis and therapy because image quality is improved.
Super-resolution techniques are quite common in astronomy and military applications [2–4] but have not been widely applied in the field of medical imaging [5].
The fundamental procedure for the creation of super-resolution images is dependent upon the following application:
-
1.
Generate a sequence of low-resolution images showing almost the same scene, whereby images are translated, rotated or scaled against each other in the subpixel dimension.
-
2.
Calculate the registration parameters between these low-resolution images with subpixel accuracy [2, 3].
-
3.
Use these registered low-resolution images for the reconstruction of one high-resolution image (super-resolution image) [2, 3].
The main question surrounding the use of super-resolution approaches for X-ray application is: How can images that are shifted against each other be obtained on X-ray systems? One easy way is to use a C-arm system and move the detector toward the X-ray source during the acquisition of a still patient. This change of the source–image–receptor distance (SID) results in a pure scaling of the images (see Fig. 1). This type of acquisition is well-suited for any kind of anatomic structure.
A different way, which is especially useful for digital subtraction angiography, is to use a common DSA sequence with contrast agent of the vascular system. Non-moving anatomical structures are eliminated by subtraction. What is left in the image is the very slightly moving (due to the blood flow) vascular system with contrast agent—this movement is sufficient to apply super-resolution techniques. Finally, the user has to select a region of interest as the movement of the complete vascular system in the images might be too complex for today’s registration algorithms. In addition the user must select a time period of the sequence where the region of interest shows a rather constant level of contrast agent.
Results and discussion
Advanced exposure control
The following general conclusions can be extracted from the optimization:
-
Detector dose can be decreased if the patient is smaller.
-
Tube current should always be as large as possible. It is better to reduce the patient dose by greater added filtration, lower tube voltage or shorter exposure time.
If the advanced exposure control is configured to maintain a constant image quality for all patient sizes, the patient dose can be reduced for newborns and children up to 30% in comparison to standard exposure controls, with constant tube voltage and constant detector dose. Furthermore, workflow with the advanced exposure control is straightforward, because the user selects an appropriate image quality. The exposure settings (tube voltage, tube current, exposure time, prefiltration) are then automatically chosen by the system.
Short pulse widths
According to Bernhardt et al. [1], the optimal exposure time can be calculated by
, where d is the object size and m is the speed of motion of the object. In pediatrics, a typical guidewire with a diameter of 0.3 mm and a speed of motion of 50 mm/s due to heart beat results in an optimal exposure time of approximately 4 ms. Hence, the exposure time of an X-ray system in pediatrics should be configurable down to 4 ms to be able to adapt the exposure control to the current workflow.
Noise-reduction algorithm
Because of basic physical principles, low-dose X-ray images contain a large amount of quantum noise. This leads to a significant reduction in the detectability of interesting structures such as small calcifications, fine vessels, guidewires and stents. In addition, noise is annoying for the user. The simplest way to reduce quantum noise is to increase dose, but this is not always appropriate. Noise reduction by image processing can lead to very similar results. In such a case, it is very important to avoid blurring the structures of interest. Differentiation between signal and noise is fundamental. Signals should be enhanced and noise smoothed; technically, that means an increase in the contrast-to-noise ratio (CNR). An example will provide better insight. Assume the signal contrast can be kept constant and the noise (standard deviation) is cut in half. This doubled CNR can alternatively be achieved by quadrupling the dose (without noise reduction by image processing).
A common technique for noise reduction is averaging over multiple consecutive images (‘temporal filtering–. Unfortunately, this method is limited to static or slow-moving image content. In cases of rapid movement, e.g., in cardiac angiography, only pure ‘spatial–image processing is applicable, i.e., every image is filtered separately.
For these spatial noise-reduction algorithms a-priori knowledge has to be applied in order to reach differentiation between signal and noise. The class of algorithms used here employs a decomposition of the incoming original image into several frequency bands. The resulting data structure is called a Laplacian pyramid [6]. These band signals are processed with filters of the same structure but with different parameter settings [7], thus reflecting the introduction of certain a-priori knowledge. The filtered band signals are collected to reconstruct the output image (Fig. 2). By appropriate parameter settings filter results can be reached, as illustrated in Fig. 3.
Finally, it should be noted that the multiscale filtering technique has the potential—besides the obvious dose-saving—of enhancing application-specific details of the image. This is very important, for example, for achieving a display of soft tissue with a high gray level differentiation.
Super-resolution technique
We give two examples of the super-resolution technique. The results for the variable SID on C-arm systems is shown in Fig. 4. Out of 31 input images (upper image in Fig. 4) available, one high-resolution (super-resolution) image was calculated. The improvement in the spatial resolution is clearly visible by means of the cut-out in the lower row of Fig. 4.
The results for the approach on DSA sequences are shown in Fig. 5. The upper image in Fig. 5 is one of the images from the original DSA sequence. The region of interest is shown by a rectangle. Because of the restriction that the vessels have to show a constant level of contrast agent, only 11 frames could be used for the calculation of the super-resolution image. In the lower row of Fig. 5, the left image shows the region of interest in the resolution of the original image. The right image is the resulting super-resolution image.
Super-resolution on DSA sequences. The upper image is one of the 11 input images. The lower row shows a magnified cut-out of one of the original input image (left) and the resulting super-resolution image (right). Areas of special interest, where the super-resolution images give additional information, are marked by circles
Conclusion
This paper presents new technologies to reduce pediatric radiation doses: advanced exposure control, short exposure times, new noise-reduction algorithms, and the super-resolution technique. All these methods offer a large potential to reduce the risks of radiation exposure.
References
Bernhardt P, Baetz L, Ruehrnschopf EP, et al (2005) Spatial frequency-dependent signal-to-noise ratio as a generalized measure of image quality. Proc SPIE 5745:407–18
Farsiu S, Robinson D, Elad M, et al (2004) Fast and robust multi-frame super-resolution. IEEE Trans Image Process 13:1327–344
Farsiu S, Robinson D, Elad M, et al (2004) Advances and challenges in super-resolution (invited paper). Int J Imag Sys Tech, Special Issue on High Resolution Image Reconstruction 14:47–7
Irani M, Peleg S (1990) Super resolution from image sequences. Proceedings of the International Conference on Pattern Recognition, Atlantic City, NJ, pp 115–20
Peled S, Yeshurun Y (2001) Superresolution in MRI: application to human white matter fiber tract visualization by diffusion tensor imaging. Magn Reson Med 45:29–5
Burt PJ, Adelson EH (1983) The Laplacian pyramid as a compact image code. IEEE Trans Comm 31:532–40; http://www.citeseer.ifi.unizh.ch/burt83laplacian.html. Cited 29 March 2006
Kunz D, Eck K, Fillbrandt H, et al (2003) A nonlinear multi-resolution gradient adaptive filter for medical images. In: Sonka M, Fitzpatrick JM (eds) Medical imaging, vol. 5032. SPIE, San Diego, pp 732–42
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Bernhardt, P., Lendl, M. & Deinzer, F. New technologies to reduce pediatric radiation doses. Pediatr Radiol 36 (Suppl 2), 212–215 (2006). https://doi.org/10.1007/s00247-006-0212-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-006-0212-4