Abstract
Tremendous advances have been made in imaging in children with both congenital and acquired heart disease. These include technical advances in cardiac catheterization and conventional angiography, especially with advancements in interventional procedures, as well as noninvasive imaging with MR and CT angiography. With rapid advances in multidetector CT (MDCT) technology, most recently 64-detector array systems (64-slice MDCT), have come a number of advantages over MR. However, both conventional and CT angiography impart radiation dose to children. Although the presence of radiation exposure to children has long been recognized, it is apparent that our ability to assess this dose, particularly in light of the rapid advancements, has been limited. Traditional methods of dosimetry for both conventional and CT angiography are somewhat cumbersome or involve a potential for substantial uncertainty. Recent developments in dosimetry, including metal oxide semiconductor field effect transistors (MOSFET) and the availability of anthropomorphic, tissue-equivalent phantoms have provided new opportunities for dosimetric assessments. Recent work with this technology in state-of-the-art cardiac angiography suites as well as with MDCT have offered direct comparisons of doses in infants and children undergoing diagnostic cardiac evaluation. It is with these dose data that assessment of risks, and ultimately the assessment of risk-benefit, can be better achieved.
Similar content being viewed by others
Introduction
There have been tremendous advances in cardiac imaging in children during the last several decades. These include technical advances for both noninvasive imaging, such as multidetector array CT (MDCT) angiography [1], and conventional angiography during heart catheterization. These advances have accelerated recently, and are part of the basis for this ALARA conference. Although these advances provide a substantial diagnostic benefit, these modalities are not without risk. Because both CT angiography and conventional angiography require ionizing radiation, this is also a shared risk. Although the bioeffects of ionizing radiation include both deterministic and stochastic effects (discussed elsewhere in this issue), the doses used for CT angiography are well below those used for deterministic effects. This is not the same for conventional angiographic assessment, where skin burns have been reported after interventional procedures [2]. The stochastic risk is in common for these two imaging modalities. Because of this, it is worth reviewing several facets of the radiation issue.
Therefore, the following material (1) briefly summarizes the rapid evolution of imaging techniques, (2) addresses whether the issue of potential radiation-induced cancer is still important, (3) discusses how traditional radiation dosimetry is problematic, (4) reviews some of the current investigations using contemporary dosimetric evaluation for both CT and conventional angiography, and (5) compares doses for both CT and conventional angiography.
Rapid evolution of imaging techniques
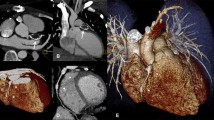
Conventional angiographic assessment during cardiac catheterization has been around for a number of decades. Some of the technical advances in fluoroscopy and angiography in children are described elsewhere in this issue (see Seibert in this issue), such as digital technology and more recently flat-panel systems. CT has also undergone a remarkable development, particularly in the last few years with the availability of 64-array MDCT (64-slice MDCT) [3]. It was not until isotropic datasets were available that multiplanar reformats and 3-D rendering became standard. Parallel to this, and equally important, was an increase in data-processing and storage capability (especially speed). These advances provided especially unique advantages for cardiovascular assessment in children, including improved monitoring, better global thoracic assessment than echocardiography or MR imaging, and decreased need for sedation, a byproduct of very fast scanning [1]. In particular, given the relatively wide effective collimation of 64-slice scanners, selective assessment of cardiovascular regions in the infant (such as the main pulmonary artery) can now be completed in less than 0.5 s. These advances with 64-slice scanners have also now made cardiac gated examinations in both infants and children possible (Fig. 1).
An infant after repair of total anomalous pulmonary venous return. Echocardiographic assessment suggested stenosis. A gated, reduced mA, CT angiography was performed at the level of the confluence of the pulmonary veins. a The upper pulmonary vein has a separate narrow (arrow) entry into the left atrium. b The remaining three pulmonary veins are confluent but also had a stenosis (arrow) at the entrance of the ventricle. This was due to a slight torque of the venous confluence that developed after an anastomosis. Based on these data, there was successful surgical correction of the stenosis
Is radiation exposure during diagnostic procedures still an important issue?
The issue of radiation dose from CT and potential development of cancer followed a series of articles in the American Journal of Roentgenology [4–6]. Although this was more than 5 years ago, the issue of potential bioeffects of low-level radiation continues to be a topical one. For example, the National Toxicology Program recently listed radiation, which includes discussion of medical radiation, as a known carcinogen [7]. Even more recently, in June 2005, the National Academy of Sciences [Biological Effects of Ionizing Radiation (BEIR) VII Report] discussed this issue of low-level radiation and potential cancer development. Among comments were the following: ‘–the risk of cancer proceeds in a linear fashion at lower doses without a threshold and –the smallest dose has the potential to cause a small increased risk to humans–[8]. Although there continues to be some debate about the risk, if any, of low-level radiation, the fact remains that a number of organizations continue to support the perspective of a potential connection. Obviously, this includes the Society for Pediatric Radiology, with this ALARA conference, but it also involves, notably, organizations such as the American Academy of Pediatrics, with a clinical report on CT and radiation [9]; the National Cancer Institute and the Society for Interventional Radiology (SIR), with a March 2005 advisory paper on radiation risks for patients and staff [10]; and the American College of Radiology Safety Committee, which is developing a White Paper on radiation risks and diagnostic imaging (David Kushner, M.D., personal communication).
In addition, the technical advances continue to, it seems, outpace our efforts at understanding the actual dose. This is particularly evident with gated cardiac examinations in children, as is discussed below.
Current dosimetry: what are the problems and solutions?
Some of the problems with MDCT include the following. First, the technology is powerful. Diagnostically, this provides us with rapid, high-quality examinations that have had marked advantages in children [1, 11]. But the word ‘powerful–also subsumes potentially higher radiation doses than were seen with conventional CT and older MDCT technology. For example, recent work with phantoms in the setting of 64-slice MDCT has shown that doses in the range of 50 mSv to nearly 120 mSv can be generated by maximizing those settings that provide radiation dose in children (Donald Frush, M.D., unpublished data). There is no provision (either from a regulatory standpoint or a manufacturer standpoint) that prevents this from occurring. Simply stated, the radiology community and manufacturers, as well as regulatory agencies, need to work toward a regulatory component of CT, such as warning indicators for certain high radiation dose examinations.
One other problem with MDCT is that the scientific community is lagging in terms of providing data on CT use. This includes outcomes for the newer MDCT as well as technical parameters for optimizing examination. These data lag behind both marketing pressures to sell the newest CT scanner and public perceptions and demands of the benefits of these types of scanners. This has been seen with screening examinations as well as gated cardiac CT studies in adults. The presumed validation of gated examinations in cardiac screening was recently promulgated by Oprah Winfrey [12]. Although the outcome of this sort of promotion has not been scientifically rigorously tested, the influence on public opinion by such spokespersons and subsequent provocative trends in use are, in general, recognized.
In addition, and perhaps most important, dose data for both the newer MDCT examinations and the contemporary angiographic equipment are lacking. This is multifactorial but in large part a result of the fact that previous methods of dosimetric assessment have been cumbersome. For example, surface assessment such as dose area product (DAP) does not provide organ dose and effective dose equivalent data for determination of risks. Traditional thermoluminescence dosimetry techniques are essentially single-exposure and take a great deal of effort to load into (and remove from) the phantoms, including anthropomorphic phantoms. Essentially, they are a single-exposure device during a CT or angiographic examination. For a CT examination, the CT dose index (CTDI) and resultant dose length product (DLP) displayed on the console are problematic for MDCT [13, 14], and in general will misrepresent the true dose to the patient. In addition, the conversion factors used with the DLP to estimate the effective dose might not reflect the dose resulting from current CT technology. Other methods of dosimetric assessment such as those using Monte Carlo codes might have not been validated using contemporary CT equipment. What is needed, then, is an accurate and simple dosimetric technology. This would allow more versatile assessment of current cardiac imaging modalities and allow for dose comparisons. This, then, would help in establishing the risk–benefit balance.
During the last few years we have been testing, validating, and applying some of this new dosimetric technology. This includes tissue-specific pediatric anthropomorphic phantoms (CIRS, Norfolk, Va.) (Fig. 2), as well as metal oxide semiconductor field effect transistor (MOSFET) technology (Thomson-Nielson, Ottawa, Canada). Briefly, organ doses supplied by MOSFET technology can be used to determine an effective dose equivalent for both CT angiography and cardiac angiography.
The first stage in the use of this combination of dosimetry tools was validation. This was carried out using traditional TLD dosimetry and Monte Carlo modeling. In adult CT examinations, MOSFET technology was found to be accurate and reliable compared with these other two more conventional techniques during chest and abdomen MDCT examinations. The advantage of the MOSFET technology is that this is a real-time assessment. Data are automatically transmitted to a laptop computer. Multiple CT examinations (or angiographic ‘runs– can be obtained in a single session, a great advantage compared with essentially single examination TLD technology.
After this validation was completed, we evaluated the MOSFET dosimetry and phantom model with CT dosimetry estimation, namely the DLP estimation. The DLP is derived from the CTDI available on scanner consoles. Using a conversion factor, the DLP estimates the dose for the MDCT parameters selected. Again, this dose does not have anything to do with the individual patient but is derived from an acrylic phantom (two sizes only) exposed using the individual settings. As mentioned before, we have found that this DLP method is relatively inaccurate in providing doses. For example, in a series of comparisons between MOSFET technology and the DLP method in adult chest and abdomen scanning, there was up to 50% greater effective dose measured by MOSFET technology than estimated by the DLP method (Lynne Hurwitz, Durham, N.C., unpublished data).
In summary, current technology provides new opportunities for dosimetric assessment in diagnostic imaging. In particular, comparison of the effective dose for pediatric cardiac angiography and CT angiography is now possible.
Dose comparisons for MDCT and conventional angiography in children
This is divided into three sections: CT angiography, gated CT angiography, and conventional angiography. Much of what is discussed here has been information recently presented at national meetings or in the press. Summary data for this material are provided.
First, MOSFET and anthropomorphic phantoms in combination were used to determine the effective dose for conventional CT angiography in both a 1- and a 5-year-old. This was performed on a 16-slice scanner (GE Healthcare, Milwaukee, Wis.). Doses using 80 kVp, 100 kVp, and 120 kVp ranged from just more than 1.0 mSv to 2.5 mSv in the 5-year old. For a 1-year-old, these doses were from about 1.5 mSv to 4.0 mSv (Fig. 3). These doses are in the range of those used for conventional (nonangiographic) chest CT in children.
A gated cardiac CT provides additional challenges in children. The nature of this examination, traditionally with relatively high tube current and very low pitch, makes it a potentially very high-dose examination. Despite this fact, excellent anatomic information can be obtained and can obviate cardiac catheterization and associated risks. Data regarding doses for pediatric gated cardiac CT, however, are limited. In our laboratory we have found that the adult dose range is approximately 11 mSv to more than 20–5 mSv; however, published data in children are lacking. We looked at gated cardiac CT in children and found, depending on the setting used, that doses could range just over 7.0 mSv to more than 25 mSv (Caroline Hollingsworth, Durham, N.C., unpublished data). Although image quality in these phantom studies was not assessed, our experience has been that acceptable image quality can be obtained with examinations performed at the lower end of these doses (Fig. 1). With that said, it is reasonable to conclude that gated cardiac CT examinations will provide doses that are probably multiples of nongated CT angiographic effective dose equivalents in children.
How do these doses compare with conventional angiography? In the literature, a number of investigations have provided dose ranges for pediatric procedures [15–21]. The range is obviously wide, and varies from approximately 5.0 mSv to more than 20 mSv, for complex interventional procedures. Again, the methodology used includes the DAP, Monte Carlo codes, and thermoluminescence dosimetry. These are, as noted previously, problematic. We have been able to use the same MOSFET and phantom technology in the pediatric catheterization laboratory in an effort to determine more reliable doses (Fig. 4).
In summary, the dose will depend on a number of factors, most obviously fluoroscopic time, and projection, use of fluoroscopy vs. cine angiography, and age and size of patient. Although it would have been possible to recreate an individual catheterization procedure, we found it more versatile to develop a dose calculator based on a dose rate (per second) depending on the projection for both cine and fluoroscopy. We found in the 1-year-old and 5-year-old that we were able to calculate effective dose equivalent for procedures. This applied to both prospective assessment for a procedure where the length of fluoroscopic and cine evaluation for the various projections could be estimated. In addition, this calculator allowed one to go back and determine a dose estimate knowing this information on a procedure that had already been performed. In general, CT angiographic (nongated) dosimetry was below conventional angiographic assessment (diagnostic only) for routine evaluation of structures such as the aorta for coarctation, or pulmonary arteries for pulmonary arteries stenosis. Again, the dose delivered will vary depending on the individual patient needs, and the physician performing the examination. Be that as it may, the calculator and the information provided from our dosimetry from MDCT offered a more versatile and accurate tool for assessment of the relative doses (for the 1- and 5-year-old children) and determination of potential risks of these procedures.
Conclusion
There has been a rapid evolution of imaging techniques. The traditional methods of dosimetry have substantial limitations for assessing radiation dose from evolving cardiac imaging modalities consisting of conventional angiography and CT angiography. However, use of MOSFET technology together with a set of pediatric anthropomorphic phantoms has resulted in an accurate and simple, extremely versatile method for radiation dose determination. In particular, this allowed us to better estimate doses for pediatric cardiac imaging during MDCT and conventional angiography. From these dosimetry tools, the effect of techniques such as decreasing tube current for gated CT examinations in small children will likely ensue, in the spirit of the ALARA principle.
References
Frush DP, Herlong RJ (2005) Pediatric thoracic CT angiography. Pediatr Radiol 35:11–5
Koenig TR, Wolff D, Mettler FA, et al (2001) Skin injuries from fluoroscopically-guided procedures. AJR 177:3–0
Frush DP (2004) Computed tomography. Advance for Imaging and Oncology Administrators 14:13–0
Brenner DJ, Elliston CD, Hall EJ, et al (2001) Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR 176:289–96
Paterson A, Frush DP, Donnelly LF (2001) Helical CT of the body: are settings adjusted for pediatric patients? AJR 176:297–01
Donnelly LF, Emery KH, Brody AS, et al (2001) Minimizing radiation dose for pediatric body applications of single-detector helical CT: strategies at a large children’s hospital. AJR 176:303–06
Department of Health and Human Services (2006) 11th report on carcinogens. Public Health Service, National Toxicology Program. http://www.ntp.niehs.nih.gov/ntp/roc/toc11.html
National Academy of Sciences (2006) Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2. National Academies Press, Washington DC. http://www.fermat.nap.edu/openbook/030909156X/html/7.html
Brody AS, Frush DP, Huda W, et al (2006) American Academy of Pediatrics clinical report: radiation risk to children from CT imaging. Pediatrics (in press)
National Cancer Institute (2006) Interventional fluoroscopy: reducing radiation risks for patients and staff. http://www.cancer.gov/cancertopics/interventionalfluoroscopy
Donnelly LF, Frush DP (2003) Pediatric multidetector body CT. Radiol Clin North Am 41:637–55
The Oprah Winfrey Show (2006) http://www.oprah.com/health/yourbody/slide/numberone/slide_yourbody_numberone_102.jhtml
Brenner DJ (2005) Letter to the editor: is it time to retire the CTDI for CT quality assurance and dose optimization? Med Phys 32:3225–226
Frush DP, Applegate K (2004) Computed tomography and radiation: understanding the issues. J Am Coll Radiol 1:113–19
Axelsson B, Khalil C, Lidegran M, et al (1999) Estimating the effective dose to children undergoing heart investigations –a phantom study. Br J Radiol 72:378–83
Schmidt PW, Dance DR, Skinner CL, et al (2000) Conversion factors for the estimation of effective dose in paediatric cardiac angiography. Phys Med Biol 45:3095–107
Campbell RM, Strieper MJ, Frias PA, et al (2005) Quantifying and minimizing radiation exposure during pediatric cardiac catheterization. Pediatr Cardiol 26:29–3
Bacher K, Bogaert E, Lapere R, et al (2005) Patient-specific dose and radiation risk estimation in pediatric cardiac catheterization. Circulation 111:83–9
Schultz FW, Geleijns J, Spoelstra FM, et al (2003) Monte Carlo calculations for assessment of radiation does to patients with congenital heart defects and to staff during cardiac catheterizations. Br J Radiol 76:638–47
Rassow J, Schmaltz AA, Hentrich F, et al (2000) Effective doses to patients from paediatric cardiac catheterization. Br J Radiol 73:172–83
Boothroyd A, McDonald E, Moores BM, et al (1997) Radiation exposure to children during cardiac catheterization. Br J Radiol 70:180–85
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Frush, D.P., Yoshizumi, T. Conventional and CT angiography in children: dosimetry and dose comparisons. Pediatr Radiol 36 (Suppl 2), 154–158 (2006). https://doi.org/10.1007/s00247-006-0190-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-006-0190-6