Abstract
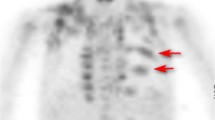
Background: Radiopharmaceutical uptake of [18F]2-deoxy-2-glucose (FDG) in brown adipose tissue is noted on 15–20% of positron emission tomography (PET) scans in children and adolescents. One report suggests that moderate-dose oral diazepam can partly or completely block FDG uptake in brown adipose tissue. Objective: To determine whether [18F]FDG uptake in brown adipose tissue can be adequately blocked by pre-medication other than moderate-dose oral diazepam. Materials and methods: One hundred and eighteen [18F]FDG PET body imaging studies were performed in 69 pediatric patients with a variety of solid tumors. The mean age at the time of imaging was 12.9 years (range 1.2–22.6 years), and 33 studies were performed in patients younger than 10 years old. Seventy-six were performed in boys and 42 in girls. Patients were imaged using a dedicated PET camera. Pre-medication was given in 88 studies: 45 received intravenous fentanyl (0.75–1.0 μg/kg), 34 received low-dose oral diazepam (0.06 mg/kg) and 9 received moderate-dose oral diazepam (0.10 mg/kg). Thirty patients received no pre-medication, 7 of whom were known to have received opiates for pain during the 12 h before the study. Six body regions in the neck and chest were reviewed for [18F]FDG uptake in brown adipose tissue. Uptake of FDG in brown fat was visually graded: 0 for no FDG uptake, 1 for low-grade uptake, 2 for moderate uptake, and 3 for intense uptake. Visual grades 2 and 3 were considered to interfere potentially with image interpretation in the neck and chest. Data were analyzed by multivariate regression using a Poisson distribution. Results: [18F]FDG uptake in brown adipose tissue was most often seen in the lateral neck region and superior and lateral to the lungs (in 36 and 39 studies, respectively). Uptake was also seen near the costovertebral junctions (15 studies), in the superior and central neck in 7 studies and in the anterior mediastinum in 2. Brown adipose tissue uptake was thought to interfere potentially with image interpretation (visual grades 2 and 3) in 19 studies—in 6 of 23 (26.1%) studies after no pre-medication and no opiates for pain, in 10 of 34 (29.4%) after low-dose oral diazepam, in 0 of 9 (0%) after moderate-dose oral diazepam, in 3 of 45 (6.7%) after intravenous fentanyl, and in 0 of 7 (0%) after opiates prescribed for pain. Intravenous fentanyl reduced the grade of brown adipose tissue compared to no drug (P=0.0039) and low-dose diazepam (P=0.0024). Low-dose diazepam had no effect when compared to no drug (P=0.984). There were inadequate data for statistical testing of moderate-dose valium and opiates prescribed for pain. Children younger than 10 years had lower uptake grades (P=0.019) than those older than 10 years. Summary: The frequency of interfering [18F]FDG uptake in brown adipose tissue is reduced by intravenous fentanyl pre-medication, which appears to be an effective alternative to the existing standard pre-medication, moderate-dose oral diazepam.




Similar content being viewed by others
References
Barrington SF, Maisey MN (1996) Skeletal muscle uptake of fluorine-18-FDG: effect of oral diazepam. J Nucl Med 37:1127–1129
Okuyama C, Sakane N, Yoshida T, et al (2002) 123I- or 125I-metaiodobenzylguanidine visualization of brown adipose tissue. J Nucl Med 43:1234–1240
Hany TF, Gharehpapagh E, Kamel EM, et al (2002) Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging 29:1393–1398
Cohade C, Osman M, Pannu HK, et al (2003) Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nucl Med 44:170–176
Yeung HWD, Grewal R, Gonen M, et al (2003) Patterns of 18F-FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. J Nucl Med 44:1789–1796
Alfonsi P, Hongnat JM, Lebrault C, et al (1995) The effects of pethidine, fentanyl and lignocaine on postanaesthetic shivering. Anaesthesia 50:214–217
Egelhoff JC, Ball WS Jr, Koch BL, et al (1997) Safety and efficacy of sedation in children using a structured sedation program. AJR 168:1250–1262
Truong MT, Erasmus JJ, Marom EM, et al (2004) Focal FDG uptake in mediastinal brown fat mimicking malignancy: a potential pitfall resolved on PET/CT. AJR 183:1127–1132
Bar-Sever Z, Keidar Z, Ben Arush WM, et al (2004) The incremental value of PET/CT over stand-alone PET in pediatric malignancies (abstract). J Nucl Med 45:136
Nanni C, Raizzini G, Halkar R, et al (2004) Initial experience in evaluating pediatric extracranial neoplastic disease with 18F-FDG (abstract). J Nucl Med 45:140
Cohade C, Mourtzikos KA, Wahl RL (2003) “USA-Fat”: prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J Nucl Med 44:1267–1270
Kawate T, Talan MI, Engel BT (1996) Sympathetic outflow to interscapular brown adipose tissue in cold-acclimated mice. Physiol Behav 59:231–223
Tatsumi M, Engles JM, Ishimori T, et al (2004) Intense 18F-FDG uptake in brown fat can be reduced pharmacologically. J Nucl Med 45:1189–1193
Kranke P, Eberhart LH, Roewer N, et al (2002) Pharmacological treatment of postoperative shivering: a quantitative systematic review of randomized controlled trials. Anesth Analg 94:453–460
Powell RM, Buggy DJ (2000) Ondansetron given before induction of anesthesia reduces shivering after general anesthesia. Anesth Analg 90:1423–1427
Horn EP, Standl T, Sessler DI, et al (1988) Physostigmine prevents postanesthetic shivering as does meperidine or clonidine. Anesthesiology 88:108–113
Garcia CA, Van Nostrand D, Majd M, et al (2004) Benzodiazepine-resistant “brown fat” pattern in positron emission tomography: two case reports of resolution with temperature control. Mol Imaging Biol 6:368–372
Acknowledgement
The authors wish to thank Norbert J. Weidner, Department of Anesthesia, Cincinnati Children’s Hospital, for information on the clinical use of drugs in post-operative shivering in children and adults.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00247-010-1860-y.
Rights and permissions
About this article
Cite this article
Gelfand, M.J., O’Hara, S.M., Curtwright, L.A. et al. Pre-medication to block [18F]FDG uptake in the brown adipose tissue of pediatric and adolescent patients. Pediatr Radiol 35, 984–990 (2005). https://doi.org/10.1007/s00247-005-1505-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-005-1505-8