Abstract
We assessed the feasibility and the impact of NAVA compared to conventional modes of mechanical ventilation in ventilatory and gas exchange parameters in post-operative children with congenital heart disease. Infants and children (age < 18 years) that underwent congenital heart surgery were enrolled. Patients were ventilated with conventional synchronized intermittent mechanical ventilation (SIMV) and subsequently transitioned to NAVA during their cardiovascular intensive care unit (CVICU) stay. The ventilatory and gas exchange parameters for the 24 h pre- and post-transition to NAVA were compared. Additional parameters assessed included pain scores and sedation requirements. Eighty-one patients met inclusion criteria with a median age of 21 days (interquartile range 13 days–2 months). The majority of patients enrolled (75.3%) had complex congenital heart disease with high surgical severity scores. The transition to NAVA was tolerated by all patients without complications. The mean peak inspiratory pressure (PIP) was 1.8 cm H2O lower (p < 0.001) and mean airway pressure (Paw) was 0.5 cm H2O lower (p = 0.009) on NAVA compared to conventional modes of mechanical ventilation. There was no significant difference in patients’ respiratory rate, tidal volume, arterial pH, pCO2, and lactate levels between the two modes of ventilation. There was a decreased sedation requirement during the time of NAVA ventilation. Comfort scores did not differ significantly with ventilator mode change. We concluded that NAVA is safe and well-tolerated mode of mechanical ventilation for our cohort of patients after congenital heart surgery. Compared to conventional ventilation there was a statistically significant decrease in PIP and Paw on NAVA.
Similar content being viewed by others
Introduction
Selection of a mode of mechanical ventilation that improves cardiopulmonary interaction and patient-ventilator synchrony is important in patients with congenital heart disease, especially in children undergoing congenital heart surgery. Positive pressure ventilation for the most part has adverse effects on the right heart. Therefore, ventilatory strategies that reduce hemodynamic load on the right ventricle including short inspiratory time, low positive end expiratory pressure (PEEP), and reduced peak inspiratory pressure (PIP) are valuable. The opposite can be said with left sided obstructive lesions where PEEP can be beneficial [1].
NAVA is a mode of mechanical ventilatory support that permits synchronized spontaneous respiratory effort with mechanical ventilation augmentation triggered by detection of an electrical signal from the diaphragm. NAVA works by transforming the neural drive into a ventilator output [2]. The ventilator-assisted breath is modulated by changes in the electrical activity of the diaphragm referred to as the Edi. The Edi is detected by electrodes inserted within the NAVA catheter, a specialized feeding tube, positioned in the esophagus at the level of the crural diaphragm [3].
Feasibility and safety studies involving the use of NAVA in neonates, infants and children have been performed within a variety of different clinical indications, including infants recovering from severe acute respiratory distress syndrome [4], neonates weighing less than 1500 g [5, 6], and infants with bronchiolitis [7]. However, experience with NAVA is limited in children following congenital heart surgery. The reported clinical trials including this patient population have been small [8,9,10,11]. Previous studies have demonstrated that when changing from conventional ventilation to NAVA, the PIP has been shown to decrease but no significant change has been previously observed in mean airway pressure (Paw) [5, 6, 10]. NAVA was introduced to our CVICU in 2011 with increased utilization over time. This is a retrospective review of our institutional experience using NAVA mode of ventilation in infants and children following congenital heart surgery. We sought to assess the feasibility and impact of NAVA compared to conventional modes of mechanical ventilation in ventilatory and gas exchange parameters in post-operative children with congenital heart disease.
Materials and Methods
We conducted a retrospective review of infants and children with CHD that underwent heart surgery at our institution during a 5-year period (January 2011 to March 2016). This study was approved by the Advocate Health Care Institutional Review Board. Due to the retrospective study design, informed consent was waived. Subjects were identified from our institutional surgical database. Eligible patients were infants and children that underwent congenital heart surgery and remained intubated in the post-operative period supported on the Servo-i ventilator (Maquet, Sweden). Patients were excluded if on multiple modes of conventional ventilation in the 24 h prior to transition to NAVA; on NAVA ventilation < 24 h; no transition to NAVA; or no arterial access.
Demographic characteristics and data from variables of interest were collected from the electronic medical record. The Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery (STAT) score, a validated surgical complexity stratification tool developed to categorize the operative mortality risk associated with congenital heart surgeries was utilized for characterization of surgical severity. STAT score category 5 corresponds with the highest risk surgical substrate. The following data were collected from the 24 h period pre- and post-transition to NAVA: all recorded ventilatory parameters (PIP, Paw, respiratory rate [RR], and tidal volume [TV]), arterial blood gas results (pH, pCO2, and lactate), sedation medications and pain scores as measured with the Face, Legs, Activity, Cry, and Consolability (FLACC) scale. These variables were recorded at varying time intervals (e.g., every 2–3 h) during the 48-h study period. A mean score for each variable was calculated for each patient and used for analysis. Sedative and analgesic medication doses (fentanyl, midazolam, dexmedetomidine) were normalized such that different types of sedation were combined and compared on the same scale using the following normalization formula: \({x_{{\text{norm}}}}=\frac{{x - {x_{{\text{min}}}}}}{{{x_{{\text{max}}}} - {x_{{\text{min}}}}}}\) [12]. Complications after transition to NAVA (e.g., trauma associated with NAVA catheter insertion, clinical worsening of respiratory and/or hemodynamic status) were assessed and recorded. Oxygen saturations and arterial partial pressure of oxygen (paO2) were not assessed in this study as the patient population had varying physiologic states and mixed cardiac lesions (i.e., patients with single versus biventricular physiology).
Summary of scalar measures were expressed by descriptive statistics (means with standard deviations, or medians with interquartile ranges) as appropriate for the data distributions. Categorical variables were summarized as frequencies and proportions. Comparison of pre- and post-NAVA parameters utilized 2-tailed paired t tests. A p value of < 0.05 was considered statistically significant.
Results
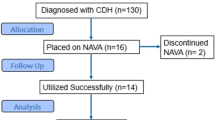
A total of 130 patients for possible study inclusion were reviewed. Of these, 81 patients met inclusion criteria in that they were mechanically ventilated in the post-operative period for a minimum of 48 h with one day of conventional ventilation followed by one day of NAVA usage (excluded patients included those with multiple forms of ventilation prior to transition to NAVA, no NAVA usage, or did not have arterial access). Fifty (62%) patients were male, the median age was 21 days (interquartile range: 13 days–2 months), and mean weight was 3.96 kg (standard deviation ± 1.73 kg). The most frequent diagnosis was hypoplastic left heart syndrome (24, 29.3%); followed by total anomalous pulmonary venous connections (6, 7.3%). The distribution of patients by STAT score category is listed in Table 1. Most of the patients were in highest risk surgical substrate. There were no complications documented related to or after the transition to NAVA for any of the patients in this sample.
Table 2 shows the comparison of mean values for measured ventilatory and arterial blood gas parameters. While on NAVA ventilation, patients demonstrated a decrease in both PIP and Paw. PIP was 1.8 cm H2O lower (p = 0.001) on NAVA compared to conventional ventilation. Paw was 0.5 cm H2O (p = 0.009) lower on NAVA compared to conventional ventilation. There was no statistical difference for patients’ respiratory rate, tidal volume, arterial pH, pCO2, and lactate levels from pre- to post- transition to NAVA. Patients were grouped according to specific mode of conventional ventilation and changes in parameters from pre- to post- transition to NAVA are reported in Table 3. Six patients were excluded from this sub-analysis since they received both modes of conventional ventilation (SIMV-PRVC and SIMV-PC) during the study period prior to the transition to NAVA. No statistical difference was found in mean airway pressure for patients transitioning from SIMV-PRVC mode of ventilation to NAVA (Table 3). Patients’ lactate levels were lower post- transition to NAVA compared to SIMV-PC ventilation (Table 3). None of the study patients experienced hemodynamic instability requiring a return to conventional ventilation.
Figure 1 compares sedation and analgesic levels pre- and post-transition to NAVA. All levels of sedation/analgesics were normalized to the same scale. Total sedation/analgesics represent the sum of all three sedation and analgesic medications. Total composite sedative/analgesic doses decreased significantly on NAVA ventilation (0.34 versus 0.24; p < 0.001). Comfort scores were low during both modes of mechanical ventilation and did not demonstrate a statistically significant difference with ventilator mode change (mean FLACC score 0.73 during conventional ventilation versus 0.8 during NAVA; p = 0.5).
Discussion
This study includes a large cohort of infants and children with complex congenital heart disease after surgical repair and palliation that were successfully transitioned from conventional modes of mechanical ventilation to NAVA. To our knowledge, this represents the largest cohort of post-operative pediatric cardiac patients where NAVA mode of ventilation has been studied.
Mechanical ventilation is frequently needed in children following congenital heart surgery. However, in the current era most of the surgical patients at our institution are fast-tracked and extubated in the operating room. The exception to this is the complex neonatal congenital heart patient that often needs longer mechanical ventilation time. These patients require not just additional ventilatory assistance but also comprehensive hemodynamic support including a variety of inotropes in order to maintain adequate cardiac output and avoid adverse cardiopulmonary interactions. Therefore, ventilatory strategies to optimize patient hemodynamics while avoiding escalation of pharmacological support are important [13]. In our institution, it has become standard practice to introduce NAVA to patients when mechanical ventilation is anticipated for more than a couple of days, if patient-ventilator asynchrony is affecting hemodynamics, or as a strategy to safely wean patients from mechanical ventilation in anticipation to transition them to non-invasive NAVA (NIV NAVA) at the time of extubation.
In the setting of anticipated prolonged mechanical ventilatory support, choosing a mode of mechanical ventilation that will provide both optimal patient-ventilator synchrony and adequate gas exchange while maintaining low peak airway pressures is essential. Furthermore, it is important to consider the patient’s cardiac anatomy and the physiologic effects of mechanical ventilation in the selection of ventilatory modes. For instance, high assist level during pressure support ventilation in single ventricle patients with cavo-pulmonary connections can impact cerebral blood flow and subsequently pulmonary blood flow. This effect can be ameliorated with NAVA ventilation [11]. Passath [14] demonstrated the physiologic effects of PEEP titration during NAVA in critically ill adults. One major objective with mechanical ventilation is always to ventilate as close to functional residual capacity (FRC) as possible without under- or over-distention of the alveoli. NAVA may be an effective tool to objectively reach FRC without directly adjusting tidal volumes or pressures levels as in conventional ventilation management but rather with intentional optimization of PEEP.
NAVA is a mode of mechanical ventilation in which the patient’s own respiratory demands determine the level of assistance; therefore, theoretically avoiding over or under assistance to the patient. Previously published studies in children and neonates have shown that NAVA is safe and feasible with reported improved patient-ventilator synchrony. The NAVA catheter is easy to insert and no significant complications have been associated with catheter insertion in the literature [8, 15, 16]. Likewise, in our study all patients tolerated transition to NAVA without reported complications associated with the catheter insertion or mode of ventilation.
Stable hemodynamics and respiratory parameters have been reported when switching from conventional ventilation to NAVA [5]. Our results correlate with these previously reported studies where transition to NAVA was tolerated without hemodynamic or respiratory deterioration as demonstrated by relatively unchanged blood gas and lactate values. In studies where cardiac patients have been included, comparable results have been observed. Zhu et al. assessed hemodynamic, oxygenation and gas exchange effects between NAVA and positive pressure ventilation in 21 infants after congenital heart surgery. The oxygenation index during NAVA ventilation was slightly improved, there was a decrease in PIP and no changes in hemodynamics were observed [11].
One of the promising advantages of NAVA is the delivery of lower peak pressures compared to other modes of mechanical ventilation [4, 6, 8, 10, 17, 18]. These previously reported studies have demonstrated that when changing from conventional ventilation to NAVA, the PIP decreases and there is a tendency towards decreased Paw [8, 16, 19]. Our study included a larger sample and found that both PIP and Paw were lower during the time of NAVA ventilation compared to conventional SIMV modes of ventilation. Subgroup analysis found that the difference in Paw for those transitioning from SIMV-PRVC modes versus NAVA was no longer statistically significant (although there was certainly a trend toward improvement present). The authors acknowledge that this effect may be confounded by the temporal relationship of surgery and anticipated lung recovery over time; however, the study design was targeting only a small amount of time during the transition to NAVA (1 day) to mitigate this confounder. Lower PIP and Paw confers several patient benefits: barotraumatic lung injury is minimized and direct impact on cardiovascular hemodynamics can be lessened.
As the trigger for NAVA is driven from the respiratory center in the brain, patient-ventilator synchrony is almost always present. Conventional ventilation historically has utilized a time trigger, pressure trigger, or most commonly a flow trigger, all with an inherent time-delay in breath initiation as compared to NAVA. This synchrony between patient and ventilator helps minimize patient discomfort and agitation, as previously reported by Piastra [4] and de la Oliva and Piastra [20]. Sedation requirements are decreased when NAVA is implemented. Lee et al. retrospectively compared NAVA to conventional ventilation in 14 chronically ventilated, trach-dependent, premature infants with bronchopulmonary dysplasia [21]. The sedation requirements, as well as the cyanotic events, were lower in the NAVA group. In our study, we found that during the time of NAVA ventilation, sedation requirements were significantly lower compared to SIMV modes of ventilation. The level of comfort did not differ between groups despite lower sedative medications used during NAVA period.
In our practice, NAVA has been found to be a very useful mode of ventilation for chronically ventilated patients with poor lung compliance. We theorize that by using NAVA we achieve better patient-ventilator synchrony and avoid the disuse atrophy of the diaphragm that is described in critically ill patients. Disuse atrophy of diaphragm fibers has been demonstrated in multiple studies when there is complete diaphragm inactivity due to prolonged mechanical ventilation with over-assistance (> 18 h) due to diaphragmatic proteolysis during inactivity [22, 23]. On several occasions, children have been transitioned from SIMV-PRVC with PIP levels in the 30–40 cm H2O range to NAVA with PIP levels decreasing immediately to < 20 cm H2O with marked improvement in gas exchange and more importantly in patient comfort. Thus, patient-ventilator synchrony can have significant implications on the care of these complex patients.
An additional advantage of NAVA in our program has been its utilization to prepare complex patients for a smooth and seamless extubation to NIV NAVA using a binasal prong interface. Wolf et al. reported their experience evaluating NAVA Edi during extubation readiness correlating neuromuscular drive to successful extubations [24]. At our institution patients meet extubation criteria when the pressure support provided by NAVA is consistently less than 10 cm H2O with stable hemodynamics. The interface is put in place prior to extubation and NIV NAVA support is initiated before tube removal while patient is being bag ventilated. It appears that the ongoing patient-ventilator synchrony provided by NIV NAVA may help reduce work of breathing and perhaps even reduce reintubation rates. This hypothesis will need to be evaluated in future studies.
This study was limited by its retrospective nature and the inherent flaws with this design. There is lack of randomization in the order of the mechanical ventilation change (NAVA always followed conventional ventilation) and perhaps the confounding variable of time between the two modes of ventilation. Due to this, we were unable to perform a matched comparison. We attempted to minimize confounding variables with temporal relationships after surgery by limiting the evaluation to a 24 h period before and after NAVA initiation only. The analyzed respiratory parameters were from data points recorded every 2–3 h by respiratory therapists and not from continuous recordings. Future studies are warranted to prospectively evaluate the differences noted in this study with matched controls.
Conclusions
NAVA was found to be safe and well-tolerated in our cohort of patients following congenital heart surgery. NAVA provided adequate gas exchange while providing decreased PIP and Paw compared to conventional modes of ventilation. Patients during NAVA ventilation consistently required less sedative medications with relative comfort scores unchanged between the two modes of ventilation perhaps due to improved synchronization. In addition, anecdotally, utilization of this technology can help prepare complex patients for safe and smooth extubations to NIV NAVA.
References
Pinsky MR, Summer WR, Wise RA et al (1983) Augmentation of cardiac function by elevation of intrathoracic pressure. J Appl Physiol 54:950–955
Sinderby C, Navalesi P, Beck J et al (1999) Neural control of mechanical ventilation. Nat Med 5:1433–1436
Sinderby C, Beck J (2007) Neurally Adjusted Ventilatory Assist (NAVA) an update and summary of experiences. Neth J Crit Care 11:243–252
Piastra M, De Luca D, Costa R et al (2014) Neurally adjusted ventilatory assist vs pressure support ventilation in infants recovering from severe acute respiratory distress syndrome: nested study. J Crit Care 29:312e1–312e5
Stein H, Howard D (2012) Neurally adjusted ventilatory assist in neonates weighing < 1500 g: a retrospective analysis. J Pediatr 160:786–789
Stein H, Alosh H, Ethington P et al (2013) Prospective crossover comparison between NAVA and pressure control ventilation in premature neonates less than 1500 grams. J Perinatol 33(6):452–456
Clement KC, Thurman Tl, Holt SJ et al (2011) Neurally triggered breaths reduce trigger delay and improve ventilator response times in ventilated infants with bronchiolitis. Intensive Care Med 37:1826–1832
Breatnach C, Conlon NP, Stack M et al (2010) A prospective crossover comparison of neurally adjusted ventilatory assist and pressure-support ventilation in a pediatric and neonatal intensive care unit population. Pediatr Crit Care Med 11:7–11
Zhu LM, Shi ZY, Ji G et al (2009) Application of neurally adjusted ventilatory assist in infants who underwent cardiac surgery for congenital heart disease. Zhongguo Dang Dai Er Ke Za Zhi 11(6):433–436
Bengtsson JA, Edberg KE (2010) Neurally adjusted ventilatory assist in children: an observational study. Pediatr Crit Care Med 11:253–257
Zhu L, Xu Z, Gong X et al (2016) Mechanical ventilation after bidirectional superior cavopulmonary anastomosis for single-ventricle physiology: a comparison of pressure support ventilation and neurally adjusted ventilatory assist. Pediatr Cardiol 37:1064–1071
Linn KA, Gaonkar B, Satterthwaite TD et al (2016) Control-group feature normalization for multivariate pattern analysis of structural MRI data using the support vector machine. NeuroImage 132:157–166
Shekerdemian L, Bohn D (1999) Cardiovascular effects of mechanical ventilation. Arch Dis Child 80:475–480
Passath C, Takala J, Tuchscherer D et al (2010) Physiologic response to changing positive end-expiratory pressure during neurally adjusted ventilator assist in sedated, critically ill adults. Chest 138(3):578–587
Duyndam A, Bol BS, Kroon A et al (2012) Neurally adjusted ventilatory assist: assessing the comfort and feasibility of use in neonates and children. Nurs Crit Care 18:86–92
Bordessoule A, Emeriaud G, Morneau S et al (2012) Neurally adjusted ventilatory assist improves patient-ventilator interaction in infants compared to conventional ventilation. Pediatr Res 72:194–202
Alander M, Peltoniemi O, Pokka T et al (2012) Comparison of pressure-, flow-, and NAVA-triggering in pediatric and neonatal ventilatory care. Pediatr Pulmonol 47:76–83
Liet JM, Barriere F, Gaillar-Le Roux B et al (2016) Physiological effects of invasive ventilation with neutrally adjusted ventilatory assist (NAVA) in a crossover study. BMC Pediatr 16(1):180–186
Brander L, Leong-Poi H, Beck J et al (2009) Titration and implementation of neurally adjusted ventilatory assist in critically ill patient. Chest 135:695–703
De la Oliva P, Schuffelmann C, Gomez-Zamora A et al (2012) Asynchrony, neural drive, ventilatory variability and COMFORT: NAVA versus pressure support in pediatric patients. A non-randomized cross-over trial. Intensive Care Med 38:838–846
Lee J, Kim HS, Jung JH et al (2017) Neurally adjusted ventilatory assist for infants under prolonged ventilation. Pediatr Int 59(5):540–544
Levine S, Nguyen T, Taylor N et al (2008) Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 358(13):1327–1335
Ducharme-Crevier L, Du Pont-Thibodeau G, Emeriaud G (2013) Interest of monitoring diaphragmatic electrical activity in the pediatric intensive care unit. Crit Care Res Pract 2013:384210
Wolf GK, Walsh BK, Green ML et al (2011) Electrical activity of the diaphragm during extubation readiness testing in critically ill children. Pediatr Crit Care Med 12(6):220–224
Acknowledgements
We would like to thank Joan Hoffman, MD, Cheryl Lefaiver, PhD, RN, and Traci Wolfe, RT, for their careful manuscript review and Bonnie Hughes, RN, BSN, CCRC for her assistance with study regulatory documents.
Author information
Authors and Affiliations
Contributions
BH helped design the study, completed data collection, assisted with result interpretation, drafted original manuscript and coordinated manuscript revisions. AM helped with data collection and reviewed manuscript. YL assisted with statistical analysis, reviewed and revised the manuscript. AVB conceptualized and designed the study, supervised data collection, provided content expertise, interpreted results, and contributed to the manuscript creation and review.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Ethical Approval
All procedures were performed in accordance with the ethical standards of Advocate Health Care’s Institutional Review Board and with the 1964 Helskinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
As per the Institutional Review Board, informed consent was waived due to the retrospective study design.
Rights and permissions
About this article
Cite this article
Baez Hernandez, N., Milad, A., Li, Y. et al. Utilization of Neurally Adjusted Ventilatory Assist (NAVA) Mode in Infants and Children Undergoing Congenital Heart Surgery: A Retrospective Review. Pediatr Cardiol 40, 563–569 (2019). https://doi.org/10.1007/s00246-018-2027-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-018-2027-0