Abstract
In patients with Long-QT Syndrome (LQTS), mechanical abnormalities have been described. Recognition of these abnormalities could potentially be used in the diagnosis of LQTS, especially in the foetus where an ECG is not available and DNA-analysis is invasive. We aimed to develop and validate a marker for these mechanical abnormalities in children and to test its feasibility in foetuses as a proof of principle. We measured the myocardial contraction duration using colour Tissue Doppler Imaging (cTDI) in 41 LQTS children and age- and gender-matched controls. Children were chosen to develop and validate the measurement of the myocardial contraction duration, due to the availability of a simultaneously recorded ECG. Feasibility of this measurement in foetuses was tested in an additional pilot study among seven LQTS foetuses and eight controls. LQTS children had a longer myocardial contraction duration compared to controls, while there was no statistical difference in heart rate. Measuring the myocardial contraction duration in children had a high inter- and intra-observer validity and reliably correlated with the QT-interval. There was an area under the curve (AUC) of 0.71, and the optimal cut-off value showed an especially high specificity in diagnosing LQTS. Measuring the myocardial contraction duration was possible in all foetuses and had a high inter- and intra-observer validity (ICC = 0.71 and ICC = 0.88, respectively). LQTS foetuses seemed to have a longer myocardial contraction duration compared to controls. Therefore, a prolonged contraction duration may be a potential marker for the prenatal diagnosis of LQTS in the future. Further studies are required to support the measurement of the myocardial contraction duration as a diagnostic approach for foetal LQTS.
Similar content being viewed by others
Introduction
Congenital Long-QT syndrome (LQTS) in an inherited cardiac repolarization disorder, with a predisposition to malignant ventricular arrhythmias, that can precipitate syncope, sudden cardiac arrest or sudden cardiac death [1]. The degree of impairment and dispersion of repolarization have been considered to play a role in the occurrence of these ventricular arrhythmias [2]. Although, patients with LQTS have a normal left ventricular function, several studies have shown mechanical abnormalities in the contraction and relaxation pattern in adult LQTS patients using M-Mode echocardiography,[3,4,5] continuous-wave Doppler images,[6] colour Tissue Doppler Imaging (cTDI) [7, 8] and Speckle Tracking [9,10,11]. One study has found mechanical dysfunction in children with the use of MRI [12]. These abnormalities were almost absent in healthy individuals, but prevalent among LQTS patients whereas in symptomatic patients this was even more frequent, suggesting their potential value in the diagnosis and risk stratification in LQTS.
The use of the presence of mechanical abnormalities in the risk stratification for malignant cardiac arrhythmias in adult and paediatric LQTS patients is perhaps more promising than its use in diagnosing LQTS, since most LQTS patients can be diagnosed based on a high clinical suspicion using electrocardiographic features, clinical and family history or in the presence of a confirmed pathogenic mutation [13]. Diagnosing LQTS prenatally is however challenging due to the lack of an accurate and comprehensible electrocardiogram (ECG), [14] the scarcity of foetal magnetocardiography, [15,16,17,18] and the invasiveness of DNA-analysis with a risk of miscarriage [19]. To date, foetal arrhythmias are diagnosed by echocardiography providing accurate information about the atrial and ventricular contractions that indirectly reflect the P-wave and QRS-complex [20]. Unfortunately echocardiography cannot assess a derivative of the QT-interval.
However, diagnosing LQTS before birth is relevant as it is a cause of sudden infant death and sudden unexplained intrauterine death [14, 21, 22]. In addition, when LQTS is diagnosed during foetal life there are important clinical consequences [14]. For instance, maternal QT-prolonging drugs frequently used during pregnancy and childbirth must be withheld, even if the mother does not suffer from LQTS. Furthermore, in case of foetal arrhythmias, some antiarrhythmic drugs that are usually given are contraindicated if the foetus has LQTS, e.g. amiodarone [23,24,25]. Lastly, a sinus bradycardia, 2:1 atrioventricular conduction or reduced beat to beat variability in the foetus can be attributed to the LQTS phenotype [26] instead of foetal distress. Hence, an accurate prenatal diagnosis of LQTS can avoid potentially unnecessary preterm deliveries or emergency caesarean sections for incorrectly presumed foetal distress.
Haugaa et al. [8] used a combination of cTDI and a simultaneously recorded ECG to evaluate the mechanical abnormalities in adult LQTS patients, and found a prolonged myocardial contraction duration in these patients. As cTDI has proven to be feasible in foetuses, has already been used in the evaluation of foetal cardiac function in various pregnancy complications,[27,28,29] and has been shown to be reliable in the measurement of foetal time intervals,[30] it could potentially be used for the measurement of the myocardial contraction duration prenatally. However, this measurement then needs to be made in the absence of an ECG.
This study had three aims: Firstly, to assess the diagnostic value of measuring the myocardial contraction duration by measuring it in children and correlating it to the QT-interval on the ECG. Children were chosen due to the availability of a simultaneously recorded ECG. Secondly, we aimed to develop a technique for the measurement of the myocardial contraction duration without the need for a simultaneously recorded ECG in children and to test its diagnostic value. Lastly, we wished to test the feasibility of this developed technique of measuring the myocardial contraction duration without a simultaneously recorded ECG in a pilot study among foetuses as a proof of principle.
Methods
Study Population (Children)
This cross-sectional study included genotype-positive LQTS children under the age of 18 years, and age- and gender-matched controls. LQTS was diagnosed based on a confirmed pathogenic mutation detected using conventional methods. Controls were referred for cardiac screening and a normal heart was confirmed after a clinical examination, ECG and echocardiography. The ECG and echocardiogram of the cases were performed during a regular follow-up visit and of the controls during a screening visit at the outpatient clinic of our hospital. The study was approved by the institutional Review Board.
Electrocardiogram (Children)
A 12-lead ECG was obtained in all subjects, either straight before or after echocardiography. All ECGs were digitalized, blinded and manually analysed by one investigator (ASV) using Image J 1.50i [National Institutes of Health, USA]. QT-intervals of three consecutive complexes were measured using the tangent method (QT-12ECG). Preceding RR-intervals were obtained (RR-12ECG), and the QTc-interval was calculated using the Bazett’s correction formula,[31] after the three consecutive complexes were averaged (QTc-12ECG).
Echocardiography (Children)
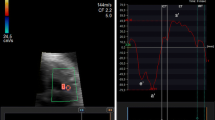
In all subjects an echocardiogram was obtained using Vivid 7 [GE, Healthcare, Horten, Norway]. The shortening fraction (SF) was calculated from the left ventricle end-diastolic and end-systolic diameters measured on M-mode. In addition, a two-dimensional guided cTDI recording was obtained by well-trained echocardiographers. All cTDI images were obtained with a frame rate of ≥ 95 frames per second from an apical four chamber view and analysed using commercially offline available software [Q-Analysis, EchoPac®, GE] averaging three cycles with the cursor on the basal interventricular septum (IVS). The IVS was chosen because of its relative easy determination in the small foetal heart. The following parameters were assessed (Fig. 1):
Schematic figure of the echocardiography parameters. Red parameters were measured unblinded for the 3-lead ECG, blue parameters were measured blinded for the 3-lead ECG. RR-3ECG RR-interval on the 3-lead ECG throughout cTDI recording, CD myocardial contraction duration measured unblinded for the surface ECG, CDblinded myocardial contraction duration measured blinded for the surface ECG, CL cycle length. s′ first-peak systolic velocity of the annulus, e′ early peak diastolic velocity, a′ late peak diastolic velocity
-
I.
The myocardial contraction duration (CD) as described by Haugaa et al. [8], defined as the time from start of R on the 3-lead ECG that was recorded throughout the cTDI procedure, to the end of the post-ejection velocity (PEV) at zero-crossing or to zero-crossing of the decreasing velocity slope in end-systole when no positive PEV was present.
-
II.
RR-intervals on the 3-lead ECG (RR-3ECG) recorded throughout the cTDI procedure.
-
III.
The myocardial contraction duration blinded for the 3-lead ECG recorded throughout the cTDI procedure (CDblinded), defined as the time from the first positive velocity slope before peak ejection velocity (zero-crossing), to the end of the PEV at zero-crossing or to zero-crossing of the decreasing velocity slope in end-systole when no positive PEV was present.
-
IV.
Cycle length (CL), defined as the time between the two successive troughs of the velocity slope in end-systole, including the CDblinded.
All parameters were measured blinded for subjects’ characteristics. It was ensured that there was sufficient time between the parameters unblinded (CD and RR-3ECG) and blinded for the 3-lead ECG recording throughout the cTDI procedure (CDblinded and CL) in order to avoid recall. One observer (ASV) measured all the parameters and these measurements were used in the primary analyses. A second observer (SC) measured the CDblinded and CL to obtain inter-observer validity and the CD, CDblinded and CL were re-measured by ASV in order to obtain intra-observer validity.
Pilot Study (Foetuses)
The feasibility of measuring the CDblinded and CL in foetuses was tested in a pilot study among foetuses with paternal or maternal genotype-positive LQTS, and healthy foetuses with a normal heart on the echocardiogram. LQTS was diagnosed after birth if the family mutation was found.
In all foetuses a two-dimensional guided cTDI recording was obtained by one foetal cardiologist (SC). The angle of insonation of the long axis of the heart was kept as small as possible, and recordings were made in the absence of foetal movement. Images were digitally saved with a frame rate of ≥ 130 frames per second. The cTDI recordings were analysed using Q-Analysis [EchoPac®, GE] averaging three cycles with the circular marker (diameter 3 mm) on the basal IVS. The CDblinded and CL were measured blinded for subjects’ characteristics by two observers (ASV and SC) to obtain inter-observer validity and a repeated analysis was done only by ASV a week later to obtain intra-observer validity.
Statistical Analysis
Clinical and echocardiographic characteristics were presented as frequencies (percentage) for categorical variables, mean (95% confidence intervals; CI) for continuous variables with an approximately symmetrical distribution and median (interquartiles) for continuous data with a skewed distribution. Differences between LQTS children and controls were analysed as appropriate by a paired t-test or a Wilcoxon signed-rank test for continuous data, and by a McNemar’s test for binary data. Differences between LQTS foetuses and controls were only presented as an absolute mean difference based on either all the first or all the last echocardiograms of the foetuses. In children, a multiple linear regression was performed to correct the CD and CDblinded for the heart rate (i.e. RR-3ECG and CL, respectively) stratified by LQTS patients or controls.
Inter-method variability in children was defined between 12-lead ECG and echocardiographic parameters, as well as between the CD and CDblinded. The inter-method variability in children and the intra- and inter-observer validity for both children and foetuses were all expressed as correlation coefficients estimated by a Pearson correlation or Spearman rank correlation test as appropriate, and the intra-class correlation coefficient (ICC) for multiple measurements based on a consistency (inter-method variability and intra-observer validity) and two-way agreement (inter-observer validity) model according to Ciccheti [32] and Fleiss [33]. Bland–Altman analyses [34] were performed to assess bias and limits of agreement for the inter-method in children and the intra- and inter-observer validity for both the children and the foetuses.
Receiver-operating characteristic (ROC) curves were constructed to determine the area under the curve (AUC) and the sensitivity and specificity for specific cut-off values of the QT-12ECG, QTc-12ECG, CD and CDblinded to identify LQTS children. The cut-off value of the QTc-interval was established at 460 ms [35] and the optimal cut-off value for both the CD and CDblinded, was defined as the Youden’s index. The reliability of the cut-off values was validated using bootstrap resampling (n = 1.000) [36].
Sampling uncertainty was quantified via 95% confidence intervals (CI) and P values. A P value < 0.05 was considered to be statistically significant. Data were analysed with R version 3.3.2 [R Foundation for Statistical Computing, Vienna, Austria].
Results
Children’s Characteristics
cTDI recordings were performed in 160 eligible subjects until April 2017. Forty-one LQTS patients could appropriately be matched to controls with respect to age and gender (Table 1). The QTc-interval was prolonged in LQTS patients (P < 0.001) with an absolute mean difference of 44 ms compared to the controls. LQTS patients were mainly diagnosed as a result of family screening (88%). There were five symptomatic LQTS patients, of whom only one had a QTc-interval > 460 ms at the time of the cTDI recording during follow-up. Of these five symptomatic patients, three patients were symptomatic before diagnosis. One had a syncopal event after waking up from a loud noise, 4 years before the data used in this study was obtained. Another patient had a syncope during cycling, within the same year as the collected ECG and echocardiogram in this study. Another patient had an event of near-drowning 2 years before the included ECG and echocardiogram. Two patients had a cardiac event after diagnosis. One patient had (pre)syncopal episodes after a nightmare, one year after our included data. In addition, there was one LQTS type 3 patient that had a sudden cardiac death during follow-up while he was treated with propranolol and mexiletine. A pacemaker and a subcutaneous implantable cardioverter defibrillator had to be explanted due to endocarditis and persistent furunculosis. The included ECG and echocardiogram were made 4 years before his death. Four asymptomatic LQTS patients had a QTc-interval > 460 ms. In all the included subjects, there was a normal SF with no difference between the LQTS patients and controls (P = 0.640). The genotypes of the LQTS patients are shown in Supplementary Table 1.
Contraction Duration in Children
The CD and CDblinded were both significantly prolonged in LQTS patients compared to controls, with an absolute mean difference of 37 ms (P = 0.005) and 39 ms (P = 0.004), respectively (Table 1). Though not statistically significant, LQTS patients had a longer RR-3ECG and CL compared to controls. However, after correction for RR-3ECG by a multiple linear regression analysis, the CD remained prolonged in LQTS patients compared to controls (P = 0.011). The same was found when the CDblinded was corrected for CL (P = 0.006).
Inter-method Variability in Children
Inter-method variability between the 12-lead ECG and echocardiographic parameters, and between the two echocardiographic parameters CD and CDblinded, are shown in Table 2. There was a high consistency between the RR-12ECG and RR-3ECG (ICC = 0.92, P < 0.001), indicating no major differences in heart rate between the 12-lead ECG and echocardiogram. In addition, there was a strong agreement between the QT-12ECG and CD (ICC = 0.87, P < 0.001). Inter-method variability between the CDblinded and the 12-lead ECG parameters showed similar results as the CD.
Comparing the two echocardiographic parameters, CD and CDblinded, showed a high consistency (ICC = 0.87, P < 0.001) with a systematic error of 9 ms and a maximal difference of 77 ms. The Bland–Altman plot is shown in Fig. 2.
Intra-and Inter-observer Validity in Children
The intra-observer validity was very high for measuring the CD (ICC = 0.96, P < 0.001) with a systematic error of 8 ms (maximal difference 46 ms). The reproducibility for the CDblinded had approximately the same magnitude as the CD (ICC = 0.94, P < 0.001) with a smaller systematic error (1 ms with a maximal difference of 52 ms). The inter-observer validity for CDblinded was also high (ICC = 0.91, P < 0.001). The intra- and inter-observer validity for CL was very high (for both ICC = 1.00 and P < 0.001) with for the intra-observer validity a systematic error of 1 ms (maximal difference 28 ms) and for the inter-observer validity a systematic error of 1 ms (maximal difference 21 ms).
Sensitivity and Specificity in Children
There was an AUC of 0.71 (95% CI by bootstrapping 0.47–0.71) for the CD and the optimal cut-off value of 422 ms (95% CI by bootstrapping 371–448 ms) yielded a sensitivity of 61% (95% CI by bootstrapping 46–76%) and a specificity of 78% (95% CI by bootstrapping 63–90%) for the diagnosis of LQTS. The CDblinded had a similar distinctiveness compared to the CD. In this relatively asymptomatic group of LQTS patients, the optimal cut-off value for the CD and CDblinded yielded a sensitivity of approximately 60% and a specificity of 78% (Table 3). In Fig. 3 the ROC curves are shown for the QT-12ECG, QTc-12ECG, CD and CDblinded.
Pilot Study in Foetuses
Fifteen foetuses (n = 7 LQTS and n = 8 controls) were included in the pilot study with a total of 24 echocardiograms (Supplementary Table 2). The CDblinded and CL could be determined in all cTDI recordings by both observers. The intra-observer validity for the determination of the CDblinded was high (r = 0.79 and ICC = 0.88 both P < 0.001) with a systematic error of 4 ms (maximal difference 69 ms) and the inter-observer validity was good (rho = 0.68, P < 0.001 and ICC = 0.71, P = 0.002) with a systematic error of 16 ms (maximal difference 87 ms). For the CL, the intra-observer validity was very high (r = 0.87 and ICC = 0.93 both P < 0.001) with a systematic error of 8 ms (maximal difference 45 ms) and the inter-observer validity was high (r = 0.74 and ICC = 0.84 both P < 0.001) with a systematic error of 11 ms (maximal difference 68 ms).
The first echocardiogram of the LQTS foetuses was compared to the first echocardiogram of the controls (to avoid including multiple measurements per foetus). There was a longer CDblinded in LQTS foetuses compared to controls with an absolute mean difference of 12 ms. The CL was also longer in LQTS patients compared to controls (∆10 ms). When the last available echocardiograms were included, there also was a longer CDblinded in LQTS foetuses compared to controls (∆35 ms) and a longer CL (∆50 ms). Figure 4 shows an example of the difference in CDblinded in foetal twins where one foetus was healthy and the other had LQTS type 3. Note that in the two right panels the configuration of the velocity curves is inverted compared to the curves in children, due to the vertex position of the foetuses.
Myocardial contraction duration by cTDI in children (both upper panels) and in foetuses (both lower panels). Note that in the two lower panels the configuration of the velocity curves is inversed compared to the curves in children, due to a different position of the foetal heart with respect to the probe of the echocardiogram
Discussion
Main Findings
This study is the first to assess abnormal ventricular contraction patterns in paediatric LQTS patients using cTDI, and shows that both the contraction duration and the contraction duration blinded for a simultaneously recorded ECG assessed by cTDI can be used in the identification of LQTS in children. In addition, measurements of the contraction duration and the contraction duration blinded for a simultaneously recorded ECG have a strong correlation and agreement with the QT-interval on a 12-lead ECG and a high reproducibility. Moreover, it is feasible to measure the contraction duration blinded for a simultaneously recorded ECG in foetuses among a broad range of gestational ages making it a potential technique for future studies on the diagnostic value of the contraction duration blinded for a simultaneously recorded ECG for the identification of foetal LQTS.
Mechanical Abnormalities in LQTS
Inhomogeneous prolongation of cardiac repolarization in LQTS patients [2] could impact on the regional mechanical cardiac function, since electrical and mechanical cardiac functions are inevitably connected via electro-mechanical coupling mechanisms [37]. Regional mechanical motion can be affected while parameters reflecting global function may still be in the normal range. High spatiotemporal resolution techniques are needed to detect such distinct alterations. With this study we showed a prolonged myocardial contraction duration in paediatric LQTS patients compared to age- and gender-matched controls using cTDI, which confirms the results of a recent study in paediatric patients by Brado et al [12] where a prolonged myocardial contraction duration was seen in paediatric LQTS patients using MRI. The prolongation of the myocardial contraction in our study is in approximately the same order of magnitude as described by Haugaa et al. [8] in adult LQTS patients using cTDI (about 40 ms) and had a concordant high inter- and intra-observer validity. None of the previous studies reported data on the diagnostic value of the presence of mechanical abnormalities.
Two cases have been reported regarding mechanical abnormalities in foetuses,[38, 39] using other modalities than cTDI (e.g. Pulsed Doppler). One of the cases was a LQTS type 3 foetus with sinus bradycardia, second-degree atrioventricular block and transient ventricular tachycardia, where a very short transmitral early deceleration time was measured by Pulsed Doppler echocardiography [38]. The authors suggested that recognition of a short mitral valve deceleration time may be helpful in the diagnosis of LQTS in the foetus. The other case was a 19-week-old LQTS type 1 foetus with a markedly prolonged left ventricular isovolumetric relaxation time on echocardiogram [39]. The measurements performed in these cases, were never validated in LQTS or correlated to QT-intervals. In a recently published multinational study, it was shown that the left ventricular isovolumetric relaxation time (LVIRT) is prolonged in LQTS foetuses, even when corrected for cycle length [40]. In our study we used a validated measurement for myocardial contraction duration, as a surrogate for QT-interval, and an approximation to the combination of the left ventricular isovolumetric relaxation time, the left ventricular contraction time and the LVIRT. LQTS foetuses seemed to have a longer myocardial contraction duration compared to controls. Unfortunately, we were not able to test this statistically due to the small sample size and unstructured collected data inherent in a pilot study.
Underlying Mechanism of Mechanical Abnormalities in LQTS
It is postulated that a deranged calcium homeostasis may play a role in the underlying mechanism of this mechanical dysfunction in LQTS [3, 4, 6,7,8, 12, 41]. A prolonged QT-interval in LQTS implies a prolonged action potential duration (APD), and this may increase the calcium influx via L-type calcium channel and a concomitant increase in the calcium-induced calcium release from the sarcoplasmic reticulum. As a consequence the calcium concentration may increase in the cytoplasm, and the duration of calcium transient may be prolonged, which can cause an impaired diastolic relaxation and a prolonged contraction duration. The increased or sustained calcium concentration could be facilitated by early afterdepolarizations, not reaching threshold for the induction of action potentials, but causing an intracellular calcium distortion [3, 4, 7, 8, 11]. Otherwise, studies on LQTS type 3 patients suggest that mechanical abnormalities may be secondary to an increased intracellular sodium concentration caused by the persistent inward sodium current [10, 41, 42].
The inhomogeneous myocardial contraction is related to either an heterogeneous distribution of mid-myocardial cells (which generally have a longer APD) [8] or ion channels [11] throughout the myocardium, and reflect electrical dispersion [5, 10, 11].
Clinical Implications and Translation to the Foetal Heart
Bearing in mind that in this study the LQTS children were mainly asymptomatic with not very long QTc-intervals, we highlight the potential of the myocardial contraction duration to detect even subtle mechanical abnormalities in paediatric LQTS patients. The myocardial contraction duration has therefore the ability to differentiate between controls and phenotype-negative LQTS children. We suggest that analysis of mechanical abnormalities might add additional information to the diagnosis in paediatric patients suspected of LQTS, used along with the QTc-interval on an ECG and provocation tests such as an exercise test or a brisk-standing test. In any case, the presence of mechanical abnormalities can be used in the risk stratification of paediatric LQTS patients as shown in previous studies [12].
The accuracy of diagnosing LQTS based on mechanical abnormalities is of a particular interest prenatally. As non-invasive tests for the diagnosis of LQTS prenatally are limited, and measuring the myocardial contraction duration in foetuses seems feasible, the contraction duration blinded for a simultaneously recorded ECG could potentially be used as a future diagnostic marker for LQTS. However, future studies should first focus on further validating measurements of the contraction duration blinded for a simultaneously recorded ECG in foetuses, and the establishment of normal ranges for healthy foetuses. Perhaps in the future, the contraction duration blinded for a simultaneously recorded ECG measured using cTDI could be used alongside the recognition of sinus bradycardia, second-degree atrioventricular block and ventricular arrhythmias in the diagnosis of foetal LQTS [26].
Limitations
This study was limited to a small sample size. Previous studies showed age- and gender-related differences in QTc-interval, [43, 44] and although there are no data available about the influences of these factors on mechanical abnormalities, it is not inconceivable that the myocardial contraction duration can be influenced by age and/or gender. For this reason LQTS children were age- and gender-matched to controls. This resulted in a small sample size but strengthened the results of our study because matching removed the potential confounding effects of age and gender. Unfortunately, we were not able to specify the myocardial contraction duration based on genotype due to this small sample size.
As mentioned, only a limited number of symptomatic LQTS patients were included in the study, and although we performed a bootstrap analysis in order to assign the accuracy of myocardial contraction duration estimates, this group will be relatively underrepresented. We were therefore not able to analyse the presence of mechanical abnormalities as a risk factor for cardiac events. Contrariwise, we aimed to develop and validate an potential marker for diagnosing foetal LQTS, and were not interested in defining risk factors for cardiac events.
The slight difference in heart rate (although not statistically significant), and the presence of beta-blocker therapy in LQTS children might influence the myocardial contraction duration. Since there is no method to correct the myocardial contraction duration for heart rate, as there is for the QT-interval on a 12-lead ECG, we corrected using a multiple linear regression. Future experiments should elucidate the exact relationship between the CD and heart rate to establish an adequate correction formula. Most of the included patients were on beta-blocker therapy according to the guidelines, [13] which did not allow us to compare patients without beta-blocker therapy to controls. However, Haugaa et al. [8] showed that the prolonged contraction duration in LQTS was not attributable to the use of beta-blocker therapy.
Conclusion
Myocardial contraction duration assessed by cTDI was prolonged in LQTS children and can be used in the identification of LQTS. Measuring the contraction duration in foetuses seems feasible, and a prolonged contraction duration may therefore be a potential marker for the prenatal diagnosis of LQTS in future. Further studies are required to support the measurement of the myocardial contraction duration as a diagnostic approach for foetal LQTS.
References
Giudicessi JR, Ackerman MJ (2013) Genotype-and phenotype-guided management of congenital long QT syndrome. Curr Probl Cardiol 38:417–455
Antzelevitch C (2007) Role of spatial dispersion of repolarization in inherited and acquired sudden cardiac death syndromes. Am J Physiol Heart Circulatory Physiol 293:H2024–H2038
Nador F, Beria G, De Ferrari GM, Stramba-Badiale M, Locati EH, Lotto A, Schwartz PJ (1991) Unsuspected echocardiographic abnormality in the long QT syndrome. Diagnostic, prognostic, and pathogenetic implications. Circulation 84:1530–1542
De Ferrari GM, Nador F, Beria G, Sala S, Lotto A, Schwartz PJ (1994) Effect of calcium channel block on the wall motion abnormality of the idiopathic long QT syndrome. Circulation 89:2126–2132
Nakayama K, Yamanari H, Otsuka F, Fukushima K, Saito H, Fujimoto Y, Emori T, Matsubara H, Uchida S, Ohe T (1998) Dispersion of regional wall motion abnormality in patients with long QT syndrome. Heart 80:245–250
ter Bekke RM, Haugaa KH, van den Wijngaard A, Bos JM, Ackerman MJ, Edvardsen T, Volders PG (2015) Electromechanical window negativity in genotyped long-QT syndrome patients: relation to arrhythmia risk. Eur Heart J 36:179–186
Savoye C, Klug D, Denjoy I, Ennezat PV, Le Tourneau T, Guicheney P, Kacet S (2003) Tissue Doppler echocardiography in patients with long QT syndrome. Eur J Echocardiogr 4:209–213
Haugaa KH, Edvardsen T, Leren TP, Gran JM, Smiseth OA, Amlie JP (2009) Left ventricular mechanical dispersion by tissue Doppler imaging: a novel approach for identifying high-risk individuals with long QT syndrome. Eur Heart J 30:330–337
Leren IS, Hasselberg NE, Saberniak J, Haland TF, Kongsgard E, Smiseth OA, Edvardsen T, Haugaa KH (2015) Cardiac mechanical alterations and genotype specific differences in subjects with long QT syndrome. JACC Cardiovasc Imaging 8:501–510
Hummel YM, Wilde AA, Voors AA, Bugatti S, Hillege HL, van den Berg MP (2013) Ventricular dysfunction in a family with long QT syndrome type 3. Europace 15:1516–1521
Haugaa KH, Amlie JP, Berge KE, Leren TP, Smiseth OA, Edvardsen T (2010) Transmural differences in myocardial contraction in long-QT syndrome: mechanical consequences of ion channel dysfunction. Circulation 122:1355–1363
Brado J, Dechant MJ, Menza M, Komancsek A, Lang CN, Bugger H, Foell D, Jung BA, Stiller B, Bode C, Odening KE (2017) Phase-contrast magnet resonance imaging reveals regional, transmural, and base-to-apex dispersion of mechanical dysfunction in patients with long QT syndrome. Heart Rhythm 14(9):1388–1397. https://doi.org/10.1016/j.hrthm.2017.04.045
Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekval TM, Spaulding C, Van Veldhuisen DJ (2015) 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 36:2793–2867
Cuneo BF, Strasburger JF, Wakai RT (2016) The natural history of fetal long QT syndrome. J Electrocardiol 49(6):807–813. https://doi.org/10.1016/j.jelectrocard.2016.07.023
Grimm B, Haueisen J, Huotilainen M, Lange S, Van Leeuwen P, Menendez T, Peters MJ, Schleussner E, Schneider U (2003) Recommended standards for fetal magnetocardiography. Pacing Clin Electrophysiol 26:2121–2126
Hamada H, Horigome H, Asaka M, Shigemitsu S, Mitsui T, Kubo T, Kandori A, Tsukada K (1999) Prenatal diagnosis of long QT syndrome using fetal magnetocardiography. Prenat Diagn 19:677–680
Cuneo BF, Strasburger JF, Yu S, Horigome H, Hosono T, Kandori A, Wakai RT (2013) In utero diagnosis of long QT syndrome by magnetocardiography. Circulation 128:2183–2191
Schneider U, Haueisen J, Loeff M, Bondarenko N, Schleussner E (2005) Prenatal diagnosis of a long QT syndrome by fetal magnetocardiography in an unshielded bedside environment. Prenat Diagn 25:704–708
Akolekar R, Beta J, Picciarelli G, Ogilvie C, D’Antonio F (2015) Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review and meta-analysis. Ultrasound Obstet Gynecol 45:16–26
Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, Cuneo BF, Huhta JC, Jonas RA, Krishnan A, Lacey S, Lee W, Michelfelder EC Sr, Rempel GR, Silverman NH, Spray TL, Strasburger JF, Tworetzky W, Rychik J (2014) Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation 129:2183–2242
Simpson JM, Maxwell D, Rosenthal E, Gill H (2009) Fetal ventricular tachycardia secondary to long QT syndrome treated with maternal intravenous magnesium: case report and review of the literature. Ultrasound Obstet Gynecol 34:475–480
Cuneo BF, Ovadia M, Strasburger JF, Zhao H, Petropulos T, Schneider J, Wakai RT (2003) Prenatal diagnosis and in utero treatment of torsades de pointes associated with congenital long QT syndrome. Am J Cardiol 91:1395–1398
Miller TE, Estrella E, Myerburg RJ, Garcia de Viera J, Moreno N, Rusconi P, Ahearn ME, Baumbach L, Kurlansky P, Wolff G, Bishopric NH (2004) Recurrent third-trimester fetal loss and maternal mosaicism for long-QT syndrome. Circulation 109:3029–3034
Oudijk MA, Gooskens RH, Stoutenbeek P, De Vries LS, Visser GH, Meijboom EJ (2004) Neurological outcome of children who were treated for fetal tachycardia complicated by hydrops. Ultrasound Obstet Gynecol 24:154–158
Bhuiyan ZA, Momenah TS, Gong Q, Amin AS, Ghamdi SA, Carvalho JS, Homfray T, Mannens MM, Zhou Z, Wilde AA (2008) Recurrent intrauterine fetal loss due to near absence of HERG: clinical and functional characterization of a homozygous nonsense HERG Q1070X mutation. Heart Rhythm 5:553–561
Cuneo BF (2015) The beginnings of long QT syndrome. Curr Opin Cardiol 30:112–117
Larsen LU, Sloth E, Petersen OB, Pedersen TF, Sorensen K, Uldbjerg N (2009) Systolic myocardial velocity alterations in the growth-restricted fetus with cerebroplacental redistribution. Ultrasound Obstet Gynecol 34:62–67
Ingul CB, Loras L, Tegnander E, Eik-Nes SH, Brantberg A (2016) Maternal obesity affects fetal myocardial function as early as in the first trimester. Ultrasound Obstet Gynecol 47:433–442
Rein AJ, O’Donnell C, Geva T, Nir A, Perles Z, Hashimoto I, Li XK, Sahn DJ (2002) Use of tissue velocity imaging in the diagnosis of fetal cardiac arrhythmias. Circulation 106:1827–1833
Herling L, Johnson J, Ferm-Widlund K, Lindgren P, Acharya G, Westgren M (2015) Automated analysis of color tissue Doppler velocity recordings of the fetal myocardium using a new algorithm. Cardiovasc Ultrasound 13:39
Bazett HC (1920) The time relations of the blood-pressure changes after excision of the adrenal glands, with some observations on blood volume changes. J Physiol 53:320–339
Cicchetti DV, Sparrow SA (1981) Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic 86:127–137
Fleiss JL (1981) Statistical methods for rates and proportions. Wiley, New York
Bland JM, Altman DG (1995) Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet 346:1085–1087
Goldman MJ (1973) Principles of clinical electrocardiography, 8 edn, Lange Medical Publications, Norwalk
Efron BTR (1986) Bootstrap methods for standard errors, confidence intervals and other methods of statistical accuracy. Stat Sci 1:54–77
Pfeiffer ER, Tangney JR, Omens JH, McCulloch AD (2014) Biomechanics of cardiac electromechanical coupling and mechanoelectric feedback. J Biomech Eng 136:021007
Sonesson SE, Eliasson H, Conner P, Wahren-Herlenius M (2014) Doppler echocardiographic isovolumetric time intervals in diagnosis of fetal blocked atrial bigeminy and 2:1 atrioventricular block. Ultrasound Obstet Gynecol 44:171–175
Tomek V, Skovranek J, Gebauer RA (2009) Prenatal diagnosis and management of fetal Long QT syndrome. Pediatr Cardiol 30:194–196
Clur SB, Vink AS, Etheridge SP, Robles de Medina PG, Rydberg A, Ackerman MJ, Wilde AA, Blom NA, Benson DW, Herberg U, Donofrio MT, Cuneo BF (2018) Left ventricular isovolumetric relaxation time is prolonged in fetal long-QT syndrome. Circulation 11:e005797
Vyas H, O’Leary PW, Earing MG, Cetta F, Ackerman MJ (2008) Mechanical dysfunction in extreme QT prolongation. J Am Soc Echocardiogr 21:e515–e517
Moss AJ, Zareba W, Schwarz KQ, Rosero S, McNitt S, Robinson JL (2008) Ranolazine shortens repolarization in patients with sustained inward sodium current due to type-3 long-QT syndrome. J Cardiovasc Electrophysiol 19:1289–1293
Vink AS, Clur SB, Geskus RB, Blank AC, De Kezel CC, Yoshinaga M, Hofman N, Wilde AA, Blom NA (2017) Effect of age and sex on the QTc interval in children and adolescents with type 1 and 2 long-QT syndrome. Circulation 10(4):e004645
Rijnbeek PR, Witsenburg M, Schrama E, Hess J, Kors JA (2001) New normal limits for the paediatric electrocardiogram. Eur Heart J 22:702–711
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declares that they have no conflict of interest.
Ethical Approval
All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Vink, A.S., Kuipers, I.M., De Bruin-Bon, R.H.A.C.M. et al. A Potential Diagnostic Approach for Foetal Long-QT Syndrome, Developed and Validated in Children. Pediatr Cardiol 39, 1413–1422 (2018). https://doi.org/10.1007/s00246-018-1911-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-018-1911-y