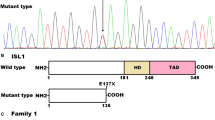
Abstract
Congenital heart defect (CHD) represents the most prevalent birth defect, and accounts for substantial morbidity and mortality in humans. Aggregating evidence demonstrates the genetic basis for CHD. However, CHD is a heterogeneous disease, and the genetic determinants underlying CHD in most patients remain unknown. In the present study, a cohort of 186 unrelated cases with CHD and 300 unrelated control individuals were recruited. The coding exons and flanking introns of the MEF2C gene, which encodes a transcription factor crucial for proper cardiovascular development, were sequenced in all study participants. The functional effect of an identified MEF2C mutation was characterized using a dual-luciferase reporter assay system. As a result, a novel heterozygous MEF2C mutation, p.R15C, was detected in an index patient with congenital double outlet right ventricle (DORV) as well as ventricular septal defect. Analysis of the proband’s pedigree showed that the mutation co-segregated with CHD with complete penetrance. The missense mutation, which changed the evolutionarily conserved amino acid, was absent in 300 control individuals. Functional deciphers revealed that the mutant MEF2C protein had a significantly decreased transcriptional activity. Furthermore, the mutation significantly reduced the synergistic activation between MEF2C and GATA4, another transcription factor linked to CHD. This study firstly associates MEF2C loss-of-function mutation with DORV in humans, which provides novel insight into the molecular pathogenesis of CHD, suggesting potential implications for genetic counseling and personalized treatment of CHD patients.




Similar content being viewed by others
References
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee (2017) Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 135:e146–e603
Postma AV, Bezzina CR, Christoffels VM (2016) Genetics of congenital heart disease: the contribution of the noncoding regulatory genome. J Hum Genet 61:13–19
Ernst MM, Marino BS, Cassedy A, Piazza-Waggoner C, Franklin RC, Brown K, Wray J (2017) Biopsychosocial predictors of quality of life outcomes in pediatric congenital heart disease. Pediatr Cardiol. https://doi.org/10.1007/s00246-017-1730-6
Kahr PC, Radke RM, Orwat S, Baumgartner H, Diller GP (2015) Analysis of associations between congenital heart defect complexity and health-related quality of life using a meta-analytic strategy. Int J Cardiol 199:197–203
Gomes-Neto M, Saquetto MB, da Silva e Silva CM, Conceição CS, Carvalho VO (2016) Impact of exercise training in aerobic capacity and pulmonary function in children and adolescents after congenital heart disease surgery: a systematic review with meta-analysis. Pediatr Cardiol 37:217–224
Morton PD, Ishibashi N, Jonas RA (2017) Neurodevelopmental abnormalities and congenital heart disease: insights into altered brain maturation. Circ Res 120:960–977
Peyvandi S, De Santiago V, Chakkarapani E, Chau V, Campbell A, Poskitt KJ, Xu D, Barkovich AJ, Miller S, McQuillen P (2016) Association of prenatal diagnosis of critical congenital heart disease with postnatal brain development and the risk of brain injury. JAMA Pediatr 170:e154450
Marelli A, Miller SP, Marino BS, Jefferson AL, Newburger JW (2016) Brain in congenital heart disease across the lifespan: the cumulative burden of injury. Circulation 133:1951–1962
Jensen AS, Idorn L, Thomsen C, von der Recke P, Mortensen J, Sørensen KE, Thilén U, Nagy E, Kofoed KF, Ostrowski SR, Søndergaard L (2015) Prevalence of cerebral and pulmonary thrombosis in patients with cyanotic congenital heart disease. Heart 101:1540–1546
Diller GP, Baumgartner H (2017) Endocarditis in adults with congenital heart disease: new answers-new questions. Eur Heart J 38:2057–2059
Kuijpers JM, Koolbergen DR, Groenink M, Peels KC, Reichert CL, Post MC, Bosker HA, Wajon EM, Zwinderman AH, Mulder BJ, Bouma BJ (2017) Incidence, risk factors, and predictors of infective endocarditis in adult congenital heart disease: focus on the use of prosthetic material. Eur Heart J 38:2048–2056
Li G, Li Y, Tan XQ, Jia P, Zhao J, Liu D, Wang T, Liu B (2017) Plasma growth differentiation factor-15 is a potential biomarker for pediatric pulmonary arterial hypertension associated with congenital heart disease. Pediatr Cardiol 38:1620–1626
Li G, Tang L, Jia P, Zhao J, Liu D, Liu B (2016) Elevated plasma connective tissue growth factor levels in children with pulmonary arterial hypertension associated with congenital heart disease. Pediatr Cardiol 37:714–721
Müller J, Heck PB, Ewert P, Hager A (2017) Noninvasive screening for pulmonary hypertension by exercise testing in congenital heart disease. Ann Thorac Surg 103:1544–1549
van der Feen DE, Bartelds B, de Boer RA, Berger RM (2017) Pulmonary arterial hypertension in congenital heart disease: translational opportunities to study the reversibility of pulmonary vascular disease. Eur Heart J 38:2034–2041
Budts W, Roos-Hesselink J, Rädle-Hurst T, Eicken A, McDonagh TA, Lambrinou E, Crespo-Leiro MG, Walker F, Frogoudaki AA (2016) Treatment of heart failure in adult congenital heart disease: a position paper of the Working Group of Grown-Up Congenital Heart Disease and the Heart Failure Association of the European Society of Cardiology. Eur Heart J 37:1419–1427
Hinton RB, Ware SM (2017) Heart failure in pediatric patients with congenital heart disease. Circ Res 120:978–994
Nandi D, Rossano JW, Wang Y, Jerrell JM (2017) Risk factors for heart failure and Its costs among children with complex congenital heart disease in a medicaid cohort. Pediatr Cardiol 38:1672–1679
Stout KK, Broberg CS, Book WM, Cecchin F, Chen JM, Dimopoulos K, Everitt MD, Gatzoulis M, Harris L, Hsu DT, Kuvin JT, Law Y, Martin CM, Murphy AM, Ross HJ, Singh G, Spray TL, American Heart Association Council on Clinical Cardiology, Council on Functional Genomics and Translational Biology, and Council on Cardiovascular Radiology and Imaging (2016) Chronic heart failure in congenital heart disease: a scientific statement from the American Heart Association. Circulation 133:770–801
Holst KA, Said SM, Nelson TJ, Cannon BC, Dearani JA (2017) Current interventional and surgical management of congenital heart disease: specific focus on valvular disease and cardiac arrhythmias. Circ Res 120:1027–1044
Khairy P (2016) Ventricular arrhythmias and sudden cardiac death in adults with congenital heart disease. Heart 102:1703–1709
Loomba RS, Aggarwal S, Gupta N, Buelow M, Alla V, Arora RR, Anderson RH (2016) Arrhythmias in adult congenital patients with bodily isomerism. Pediatr Cardiol 37:330–337
Lüscher TF (2016) Frontiers in congenital heart disease: pulmonary hypertension, heart failure, and arrhythmias. Eur Heart J 37:1407–1409
McLeod CJ, Warnes C (2016) Recognition and management of arrhythmias in adult congenital heart disease. Curr Opin Cardiol 31:117–123
Diller GP, Baumgartner H (2016) Sudden cardiac death during exercise in patients with congenital heart disease: the exercise paradox and the challenge of appropriate counselling. Eur Heart J 37:627–629
Diller GP, Kempny A, Alonso-Gonzalez R, Swan L, Uebing A, Li W, Babu-Narayan S, Wort SJ, Dimopoulos K, Gatzoulis MA (2015) Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow-up at a large tertiary center. Circulation 13:2118–2125
Engelings CC, Helm PC, Abdul-Khaliq H, Asfour B, Bauer UM, Baumgartner H, Kececioglu D, Körten MA, Diller GP, Tutarel O (2016) Cause of death in adults with congenital heart disease—an analysis of the German National Register for Congenital Heart Defects. Int J Cardiol 211:31–36
Jortveit J, Eskedal L, Hirth A, Fomina T, Døhlen G, Hagemo P, Tell GS, Birkeland S, Øyen N, Holmstrøm H (2016) Sudden unexpected death in children with congenital heart defects. Eur Heart J 37:621–626
Koyak Z, de Groot JR, Bouma BJ, Zwinderman AH, Silversides CK, Oechslin EN, Budts W, Van Gelder IC, Mulder BJ, Harris L (2017) Sudden cardiac death in adult congenital heart disease: can the unpredictable be foreseen? Europace 19:401–406
Bouma BJ, Mulder BJ (2017) Changing landscape of congenital heart disease. Circ Res 120:908–922
Mandalenakis Z, Rosengren A, Skoglund K, Lappas G, Eriksson P, Dellborg M (2017) Survivorship in children and young adults with congenital heart disease in Sweden. JAMA Int 177:224–230
Williams RG (2016) Late causes of death after congenital heart defects: a population-based study from Finland. J Am Coll Cardiol 68:499–501
Andersen TA, Troelsen Kde L, Larsen LA (2014) Of mice and men: molecular genetics of congenital heart disease. Cell Mol Life Sci 71:1327–1352
Blue GM, Kirk EP, Giannoulatou E, Sholler GF, Dunwoodie SL, Harvey RP, Winlaw DS (2017) Advances in the genetics of congenital heart disease: a clinician’s guide. J Am Coll Cardiol 69:859–870
Edwards JJ, Gelb BD (2016) Genetics of congenital heart disease. Curr Opin Cardiol 31:235–241
Fahed AC, Gelb BD, Seidman JG, Seidman CE (2013) Genetics of congenital heart disease: the glass half empty. Circ Res 112:707–720
Boyle L, Wamelink MM, Salomons GS, Roos B, Pop A, Dauber A, Hwa V, Andrew M, Douglas J, Feingold M, Kramer N, Saitta S, Retterer K, Cho MT, Begtrup A, Monaghan KG, Wynn J, Chung WK (2016) Mutations in TKT are the cause of a syndrome including short stature, developmental delay, and congenital heart defects. Am J Hum Genet 98:1235–1242
Cao Y, Wang J, Wei C, Hou Z, Li Y, Zou H, Meng M, Wang W, Jiang L (2016) Genetic variations of NKX2-5 in sporadic atrial septal defect and ventricular septal defect in Chinese Yunnan population. Gene 575:29–33
Chen J, Qi B, Zhao J, Liu W, Duan R, Zhang M (2016) A novel mutation of GATA4 (K300T) associated with familial atrial septal defect. Gene 575:473–477
Ellesøe SG, Johansen MM, Bjerre JV, Hjortdal VE, Brunak S, Larsen LA (2016) Familial atrial septal defect and sudden cardiac death: identification of a novel NKX2-5 mutation and a review of the literature. Congenit Heart Dis 11:283–290
Huang RT, Wang J, Xue S, Qiu XB, Shi HY, Li RG, Qu XK, Yang XX, Liu H, Li N, Li YJ, Xu YJ, Yang YQ (2017) TBX20 loss-of-function mutation responsible for familial tetralogy of Fallot or sporadic persistent truncus arteriosus. Int J Med Sci 14:323–332
Huang RT, Xue S, Wang J, Gu JY, Xu JH, Li YJ, Li N, Yang XX, Liu H, Zhang XD, Qu XK, Xu YJ, Qiu XB, Li RG, Yang YQ (2016) CASZ1 loss-of-function mutation associated with congenital heart disease. Gene 595:62–68
Li FF, Deng X, Zhou J, Yan P, Zhao EY, Liu SL (2016) Characterization of human bone morphogenetic protein gene variants for possible roles in congenital heart disease. Mol Med Rep 14:1459–1464
Li L, Wang J, Liu XY, Liu H, Shi HY, Yang XX, Li N, Li YJ, Huang RT, Xue S, Qiu XB, Yang YQ (2017) HAND1 loss-of-function mutation contributes to congenital double outlet right ventricle. Int J Mol Med 39:711–718
Li N, Subrahmanyan L, Smith E, Yu X, Zaidi S, Choi M, Mane S, Nelson-Williams C, Bahjati M, Kazemi M, Hashemi M, Fathzadeh M, Narayanan A, Tian L, Montazeri F, Mani M, Begleiter ML, Coon BG, Lynch HT, Olson EN, Zhao H, Ruland J, Lifton RP, Mani A (2016) Mutations in the histone modifier PRDM6 are associated with isolated nonsyndromic patent ductus arteriosus. Am J Hum Genet 98:1082–1091
Li RG, Xu YJ, Wang J, Liu XY, Yuan F, Huang RT, Xue S, Li L, Liu H, Li YJ, Qu XK, Shi HY, Zhang M, Qiu XB, Yang YQ (2017) GATA4 loss-of-function mutation and the congenitally bicuspid aortic valve. Am J Cardiol. https://doi.org/10.1016/j.amjcard.2017.11.012
Li X, Shi L, Xu M, Zheng X, Yu Y, Jin J (2017) RCAN1 mutation and functional characterization in children with sporadic congenital heart disease. Pediatr Cardiol. https://doi.org/10.1007/s00246-017-1746-y
Li YJ, Yang YQ (2017) An update on the molecular diagnosis of congenital heart disease: focus on loss-of-function mutations. Expert Rev Mol Diagn 17:393–401
Liu D, Liu QQ, Guan LH, Jiang X, Zhou DX, Beghetti M, Qu JM, Jing ZC (2016) BMPR2 mutation is a potential predisposing genetic risk factor for congenital heart disease associated pulmonary vascular disease. Int J Cardiol 211:132–136
Liu S, Su Z, Tan S, Ni B, Pan H, Liu B, Wang J, Xiao J, Chen Q (2017) Functional analyses of a novel CITED2 nonsynonymous mutation in Chinese Tibetan patients with congenital heart disease. Pediatr Cardiol 38:1226–1231
Lu CX, Gong HR, Liu XY, Wang J, Zhao CM, Huang RT, Xue S, Yang YQ (2016) A novel HAND2 loss-of-function mutation responsible for tetralogy of Fallot. Int J Mol Med 37:445–451
Priest JR, Osoegawa K, Mohammed N, Nanda V, Kundu R, Schultz K, Lammer EJ, Girirajan S, Scheetz T, Waggott D, Haddad F, Reddy S, Bernstein D, Burns T, Steimle JD, Yang XH, Moskowitz IP, Hurles M, Lifton RP, Nickerson D, Bamshad M, Eichler EE, Mital S, Sheffield V, Quertermous T, Gelb BD, Portman M, Ashley EA (2016) De novo and rare variants at multiple loci support the oligogenic origins of atrioventricular septal heart defects. PLoS Genet 12:e100596
Qiao XH, Wang F, Zhang XL, Huang RT, Xue S, Wang J, Qiu XB, Liu XY, Yang YQ (2017) MEF2C loss-of-function mutation contributes to congenital heart defects. Int J Med Sci 14:1143–1153
Qiao XH, Wang Q, Wang J, Liu XY, Xu YJ, Huang RT, Xue S, Li YJ, Zhang M, Qu XK, Li RG, Qiu XB, Yang YQ (2017) A novel NR2F2 loss-of-function mutation predisposes to congenital heart defect. Eur J Med Genet. https://doi.org/10.1016/j.ejmg.2017.12.003
Shanshen E, Rosenberg J, Van Bergen AH (2017) Identification of novel congenital heart disease candidate genes using chromosome microarray. Pediatr Cardiol. https://doi.org/10.1007/s00246-017-1741-3
Shi LM, Tao JW, Qiu XB, Wang J, Yuan F, Xu L, Liu H, Li RG, Xu YJ, Wang Q, Zheng HZ, Li X, Wang XZ, Zhang M, Qu XK, Yang YQ (2014) GATA5 loss-of-function mutations associated with congenital bicuspid aortic valve. Int J Mol Med 33:1219–1226
Sifrim A, Hitz MP, Wilsdon A, Breckpot J, Turki SH, Thienpont B, McRae J, Fitzgerald TW, Singh T, Swaminathan GJ, Prigmore E, Rajan D, Abdul-Khaliq H, Banka S, Bauer UM, Bentham J, Berger F, Bhattacharya S, Bu’Lock F, Canham N, Colgiu IG, Cosgrove C, Cox H, Daehnert I, Daly A, Danesh J, Fryer A, Gewillig M, Hobson E, Hoff K, Homfray T, INTERVAL Study, Kahlert AK, Ketley A, Kramer HH, Lachlan K, Lampe AK, Louw JJ, Manickara AK, Manase D, McCarthy KP, Metcalfe K, Moore C, Newbury-Ecob R, Omer SO, Ouwehand WH, Park SM, Parker MJ, Pickardt T, Pollard MO, Robert L, Roberts DJ, Sambrook J, Setchfield K, Stiller B, Thornborough C, Toka O, Watkins H, Williams D, Wright M, Mital S, Daubeney PE, Keavney B, Goodship J, UK10K Consortium, Abu-Sulaiman RM, Klaassen S, Wright CF, Firth HV, Barrett JC, Devriendt K, FitzPatrick DR, Brook JD, Deciphering Developmental Disorders Study, Hurles ME (2016) Distinct genetic architectures for syndromic and nonsyndromic congenital heart defects identified by exome sequencing. Nat Genet 48:1060–1065
Sun YM, Wang J, Qiu XB, Yuan F, Li RG, Xu YJ, Qu XK, Shi HY, Hou XM, Huang RT, Xue S, Yang YQ (2016) A HAND2 loss-of-function mutation causes familial ventricular septal defect and pulmonary stenosis. G3 6:987–992
Sun YM, Wang J, Qiu XB, Yuan F, Xu YJ, Li RG, Qu XK, Huang RT, Xue S, Yang YQ (2016) PITX2 loss-of-function mutation contributes to tetralogy of Fallot. Gene 577:258–264
Tong YF (2016) Mutations of NKX2.5 and GATA4 genes in the development of congenital heart disease. Gene 588:86–94
Wang F, Wang H, Wang L, Zhou S, Chang M, Zhou J, Dou Y, Wang Y, Shi X (2016) Association between single nucleotide polymorphisms in NFATC1 signaling pathway genes and susceptibility to congenital heart disease in the Chinese population. Pediatr Cardiol 37:1548–1561
Wang J, Hu XQ, Guo YH, Gu JY, Xu JH, Li YJ, Li N, Yang XX, Yang YQ (2017) HAND1 loss-of-function mutation causes tetralogy of Fallot. Pediatr Cardiol 38:547–557
Wang J, Mao JH, Ding KK, Xu WJ, Liu XY, Qiu XB, Li RG, Qu XK, Xu YJ, Huang RT, Xue S, Yang YQ (2015) A novel NKX2.6 mutation associated with congenital ventricular septal defect. Pediatr Cardiol 36:646–656
Wang X, Chang WL, Chen CA, Rosenfeld JA, Al Shamsi A, Al-Gazali L, McGuire M, Mew NA, Arnold GL, Qu C, Ding Y, Muzny DM, Gibbs RA, Eng CM, Walkiewicz M, Xia F, Plon SE, Lupski JR, Schaaf CP, Yang Y (2017) Germline mutations in ABL1 cause an autosomal dominant syndrome characterized by congenital heart defects and skeletal malformations. Nat Genet 49:613–617
Werner P, Latney B, Deardorff MA, Goldmuntz E (2016) MESP1 mutations in patients with congenital heart defects. Hum Mutat 37:308–314
Xie X, Shi X, Xun X, Rao L (2016) Association of NKX2-5 genetic polymorphisms with the risk of congenital heart disease: a meta-analysis. Pediatr Cardiol 37:953–961
Xu YJ, Qiu XB, Yuan F, Shi HY, Xu L, Hou XM, Qu XK, Liu X, Huang RT, Xue S, Yang YQ, Li RG (2017) Prevalence and spectrum of NKX2.5 mutations in patients with congenital atrial septal defect and atrioventricular block. Mol Med Rep 15:2247–2254
Yoshida A, Morisaki H, Nakaji M, Kitano M, Kim KS, Sagawa K, Ishikawa S, Satokata I, Mitani Y, Kato H, Hamaoka K, Echigo S, Shiraishi I, Morisaki T (2016) Genetic mutation analysis in Japanese patients with non-syndromic congenital heart disease. J Hum Genet 61:157–162
Zhang M, Li FX, Liu XY, Huang RT, Xue S, Yang XX, Li YJ, Liu H, Shi HY, Pan X, Qiu XB, Yang YQ (2017) MESP1 loss of function mutation contributes to double outlet right ventricle. Mol Med Rep 16:2747–2754
Zhao CM, Sun B, Song HM, Wang J, Xu WJ, Jiang JF, Qiu XB, Yuan F, Xu JH, Yang YQ (2016) TBX20 loss-of-function mutation associated with familial dilated cardiomyopathy. Clin Chem Lab Med 54:325–332
Zheng GF, Wei D, Zhao H, Zhou N, Yang YQ, Liu XY (2012) A novel GATA6 mutation associated with congenital ventricular septal defect. Int J Mol Med 29:1065–1071
Zhou YM, Dai XY, Huang RT, Xue S, Xu YJ, Qiu XB, Yang YQ (2016) A novel TBX20 loss of function mutation contributes to adult onset dilated cardiomyopathy or congenital atrial septal defect. Mol Med Rep 14:3307–3314
Zhou YM, Dai XY, Qiu XB, Yuan F, Li RG, Xu YJ, Qu XK, Huang RT, Xue S, Yang YQ (2016) HAND1 loss-of-function mutation associated with familial dilated cardiomyopathy. Clin Chem Lab Med 54:1161–1167
McCulley DJ, Black BL (2012) Transcription factor pathways and congenital heart disease. Curr Top Dev Biol 100:253–277
Lin Q, Schwarz J, Bucana C, Olson EN (1997) Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science 276:1404–1407
Barnes RM, Harris IS, Jaehnig EJ, Sauls K, Sinha T, Rojas A, Schachterle W, McCulley DJ, Norris RA, Black BL (2016) MEF2C regulates outflow tract alignment and transcriptional control of Tdgf1. Development 143:774–779
Morin S, Charron F, Robitaille L, Nemer M (2000) GATA-dependent recruitment of MEF2 proteins to target promoters. EMBO J 19:2046–2055
Dong C, Yang XZ, Zhang CY, Liu YY, Zhou RB, Cheng QD, Yan EK, Yin DC (2017) Myocyte enhancer factor 2C and its directly-interacting proteins: a review. Prog Biophys Mol Biol 126:22–30
Pan Y, Wang ZG, Liu XY, Zhao H, Zhou N, Zheng GF, Qiu XB, Li RG, Yuan F, Shi HY, Hou XM, Yang YQ (2015) A novel TBX1 loss-of-function mutation associated with congenital heart disease. Pediatr Cardiol 36:1400–1410
Guo DF, Li RG, Yuan F, Shi HY, Hou XM, Qu XK, Xu YJ, Zhang M, Liu X, Jiang JQ, Yang YQ, Qiu XB (2016) TBX5 loss-of-function mutation contributes to atrial fibrillation and atypical Holt-Oram syndrome. Mol Med Rep 13:4349–4356
Rocha H, Sampaio M, Rocha R, Fernandes S, Leão M (2016) MEF2C haploinsufficiency syndrome: report of a new MEF2C mutation and review. Eur J Med Genet 59:478–482
Yuan F, Qiu ZH, Wang XH, Sun YM, Wang J, Li RG, Liu H, Zhang M, Shi HY, Zhao L, Jiang WF, Liu X, Qiu XB, Qu XK, Yang YQ (2017) MEF2C loss-of-function mutation associated with familial dilated cardiomyopathy. Clin Chem Lab Med. https://doi.org/10.1515/cclm-2017-0461
Kodo K, Nishizawa T, Furutani M, Arai S, Ishihara K, Oda M, Makino S, Fukuda K, Takahashi T, Matsuoka R, Nakanishi T, Yamagishi H (2012) Genetic analysis of essential cardiac transcription factors in 256 patients with non-syndromic congenital heart defects. Circ J 76:1703–1711
Acknowledgements
We sincerely thank the study subjects for their dedication to the research. This work was financially supported by grants from the National Natural Science Foundation of China (81470372) and the Natural Science Foundation of Shanghai, China (16ZR1432500).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Electronic Supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, CX., Wang, W., Wang, Q. et al. A Novel MEF2C Loss-of-Function Mutation Associated with Congenital Double Outlet Right Ventricle. Pediatr Cardiol 39, 794–804 (2018). https://doi.org/10.1007/s00246-018-1822-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-018-1822-y