Abstract
17α-ethinylestradiol (EE2) is a potent synthetic estrogen that is routinely detected in aquatic ecosystems and exhibits estrogenic activity. Acute and chronic toxicity have been described for oviparous and ovoviviparous fish species; however, no information is available on the impacts of EE2 on viviparous, matrotrophic fish despite their ecological importance. The present study investigated the consequences of long-term EE2 exposure in the least killifish (Heterandria formosa). Effects on growth, time-to-sexual maturity, fecundity, and offspring survival were examined in an 8-month, life-cycle experiment. Starting as 0–6-day-old fish, least killifish were continuously exposed to EE2 at nominal concentrations of 0, 5, or 25 ng/L (measured concentrations averaged 0, 4.3, and 21.5 ng/L respectively). In the F0 generation, EE2-exposure did not affect survival but resulted in increased time-to-sexual maturity and a sex-dependent effect on size; female standard length was reduced while male standard length was increased. This caused the ordinarily larger females and smaller males to become more similar in size. Condition factor was reduced for both sexes. Fecundity was reduced by 50% and 75% at 5 and 25 ng/L EE2-exposure respectively. Continued EE2-exposure in the F1 generation resulted in significantly reduced survival. These results suggest that despite their matrotrophic development, these fish experience delayed development and reduced reproductive success from EE2-exposure and that effects appear to intensify in the second generation.
Similar content being viewed by others
More than 3000 pharmaceutical compounds are in use as human and veterinary medicines (Diaz-Cruz and Barcelo’ 2004; Williams 2005). The worldwide and year-round use of pharmaceuticals often result in substantial releases into the environment (Buchberger 2007; Vieno et al. 2007; World Health Organization (WHO) 2012). Because pharmaceuticals were designed to have specific pharmacological effects in humans and veterinary animals, the fact that many of the targeted hormonal and metabolic systems are conserved in all vertebrates means that pharmacological effects also may occur in aquatic animals, such as fish and frogs (Kah and Dufour 2011; Liu et al. 2012a; Trudeau et al. 2005; Wang et al. 2011).
One pharmaceutical for which there is environmental concern is 17α-ethinylestradiol (EE2). It has a high potency, high environmental persistence, and strong tendencies for bioaccumulation and bioconcentration (Al-Ansari et al. 2010; Gibson et al. 2005; Liu et al. 2012a). It is a synthetic derivative of the natural estrogen estradiol and is used in combination with other steroid hormones as the active ingredient in most contraceptives. In addition, it is used for treatment of breast and prostate cancers, female hypogonadism, and vasomotor symptoms affiliated with menopause (Hardman et al. 1996; Langer 2009; Shifren and Schiff 2010). In agriculture, EE2 is used to control ovulation and treat reproductive disorders in livestock (Gadd et al. 2010; Liu et al. 2012b; Ying et al. 2002). Consequently, it is released into the aquatic environment directly via sewage treatment plants (STPs), as well as via runoff from agricultural lands used for breeding cattle, lands fertilized with STP sludge or livestock waste, or lands irrigated with STP effluent (Xu et al. 2009; Yang et al. 2012). While a substantial proportion (34% to 90%) of EE2 is removed in STPs during the treatment process, EE2 concentrations of < 0.1 to 14 ng/L have been reported for STP effluents (Cargouet et al. 2004; Clouzot et al. 2008; Kostich et al. 2013; Schwindt et al. 2014; Zhang et al. 2011). Similarly, EE2 concentrations of 17 to 55 ng/L were reported for runoff from a field in irrigated with STP effluent (Xu et al. 2009; Yang et al. 2012). As a consequence of these multiple sources of EE2, surface water concentrations typically range from 0 to 35.6 ng/L (Belfroid et al. 1999; Lei et al. 2009; Snyder et al. 1999; Ying et al. 2009; Zou et al. 2013). Because EE2 has an environmental half-life of 10 to 81 days, it can be found in surface waters at a substantial distance from the original entry points (Barel-Cohen et al. 2006; Johnson 2010; Jurgens et al. 2002; Nelson et al. 2011; Pojana et al. 2007; Ying et al. 2002). EE2 is an endocrine-disrupting chemical (EDC) and EE2 exposure has been correlated with decreased fertility in wildlife, as well as intersex and reduced fertilization success in amphibians (Hemming et al. 2002; Salla et al. 2016; Tamschick et al. 2016). In fish, exposure to EE2 has been linked to a variety of negative effects, including decreased fertilization success, decreased growth, altered anatomical features, inhibited gamete maturation, reduced gonad growth, decreased gamete production (Chen et al. 2010; Duong et al. 2010; Dziewecyznski and Hebert 2013; Saaristo et al. 2009; Silva et al. 2012; Sumpter 2005), vitellogenin induction, altered secondary sexual characteristics, intersex in male fishes, female-biased sex ratios (Bizarro et al. 2014; Lange et al. 2011; Lavelle and Sorensen 2011; Sole’ et al. 2003; Van Aerle et al. 2001), reduced embryo viability (Bhandari et al. 2015; Gennotte et al. 2015; Nash et al. 2004; Segner et al. 2003), decreased fecundity (Balch et al. 2004; Montgomery et al. 2012; Schwindt et al. 2014; Teta and Naik 2016), atypical reproductive behavior, reduced offspring survival, and population-level declines (Bhandari et al. 2015; Johnson and Sumpter 2014; Kidd et al. 2007; Kristensen et al. 2005; Nash et al. 2004; Schwindt et al. 2014).
Most of this information on EE2 effects in fish is for oviparous (egg-laying) fish, whereas insights are substantially more limited for viviparous (live-bearing) fish and nonexistent for live-bearing matrotrophic fish. The reproductive strategy is likely to be of significance with respect to the effects of EE2 because of the large differences in the environment where fertilization and early development occurs (inside the female in viviparous fish versus the external environment in oviparous fish) and the source of the nutrition (e.g., egg yolk in lecithotrophic fish vs. directly from the mother and a reduced role of vitellogenesis in matrotrophic fish). The role of estrogen (and therefore the influence of the estrogenic endocrine disruptor EE2) also differs among fish with different reproductive strategies. For example, estrogen plays a key role in sexual behavior, including inducing and maintaining female receptivity in viviparous fish, such as the guppy Poecilia reticulata (Kolpin et al. 2002; Kristensen et al. 2005), whereas prostaglandins are the hormonal trigger for female sexual behavior in many oviparous fish, such as goldfish and medaka (Kitamura et al. 1994; Sorensen and Goetz 1993; Stacey and Peter 1979). Finally, reproductive strategy is likely to affect the long-term consequences of EE2 exposure, because population-level outcomes of EE2’s influence on factors, such as fecundity, are bound to differ between a fish that lays thousands of eggs (an r-selected species) versus one that produces a few young (a K-selected species).
The purpose the present study was to investigate the impacts of EE2 exposures in the viviparous, matrotrophic least killifish (Heterandria formosa). This member of the topminnow family, Poeciliida is found in a variety of habitats throughout the coastal plain of the southeastern United States (Fraser and Renton 1940). Females exhibit extreme matrotrophy and a high degree of superfetation—a phenomenon in which multiple broods of developing embryos, each brood at a different stage of development, occur simultaneously within the ovary of a single female (Travis et al. 1987; Turner 1937). Fertilization is internal, and sperm can be stored in the female’s ovary for more than 10 months (Constanz 1989; Rosen and Bailey 1963). They have a gestation period of 27 to 35 days (Fraser and Renton 1940; Scrimshaw 1944), and fertilized eggs are retained within the ovary until the embryos are fully developed and large enough to be self-sufficient and free swimming (Rosen and Bailey 1963). Females produce one to ten offspring per brood, and superfetation allows the females to have successive broods with intervals as short as a few days (Cheong et al. 1984; Reznick et al. 1996; Travis et al. 1987). Least killifish are sexually dimorphic with females and are typically larger than males; adult total length averages 27 and 15 mm, respectively (Belk and Lydeard 1994; Fraser and Renton 1940). In our laboratory cultures, fish reached sexual maturity in approximately 3 months. We used chronic lifetime EE2 exposures that started shortly after birth of the fish and continued into the next generation. Responses monitored included growth, sexual development, and fecundity in the F0 generation and survival in the F1 generation.
Materials and Methods
Fish Collection and Maintenance
Breeding stocks were established with wild caught adult least killifish from Lake Martin near St. Martinville, LA (30.331°N, 91.905°W), a cypress and tupelo swamp. The initial population was established with approximately 1500 individuals. Fish were housed in a greenhouse at the University of Louisiana’s Ecology Center, under natural light conditions in a 568-L tank system (consisting of 2 trays with a shared filter tank) with recirculating local well water. Young fish were fed once daily with newly hatched brine shrimp larvae (O.S.I. Pro 80; Ocean Star International); juveniles and adults were fed daily alternating between brine shrimp larvae and commercial fish flakes (TetraMin Tropical Flakes; Tetra Holding). Water temperatures ranged from 12.8 to 37 °C (reflecting greenhouse conditions and representative of natural variation in water temperature in southern Louisiana); the pH ranged from 6.9 to 7.2. The fish were collected under a collecting permit from the Louisiana Department of Wildlife and Fisheries. Fish maintenance and research protocols were approved by the University of Louisiana at Lafayette Institutional Animal Care and Use Committee (IACUC approval number: 2011-8717-040r).
Test Chemical
Stock solutions of EE2 (17α-Ethinylestradiol, > 98% purity, obtained from Sigma Aldrich, CAS# 57-63-6) were prepared using ethanol (laboratory-grade; Thermo-Fisher Scientific) as the solvent. The nominal EE2 exposure concentrations were as follows: 0 (solvent control), 5 ng/L, or 25 ng/L EE2. Ethanol concentration was established in working solutions at 0.7 μl/ml (0.002%) in all treatments, including control. A clean-water control was not used, because preliminary experiments in our lab did not reveal any differences in growth, reproduction, sex ratio, survival, or offspring viability between clean-water controls and solvent controls. Our experimental concentrations were chosen, because studies have typically reported that EE2 concentrations in receiving waters range from 0.1 to 68 ng/L (Christiansen et al. 2002; Kunz et al. 2015; Schultz et al. 2003; Sun et al. 2009; Zou et al. 2013). Standard solutions for EE2 were prepared in accordance to the methods used in Sarat and Rambau (Sarat and Rambabu (2012).
Experimental Setup
Young fish (0–6 days old) were randomly selected from our least killifish breeding population at the Ecology Center (EC). Thirty replicates with two fish each were exposed for each of the three EE2 treatments (0, 5, and 25 ng/L) in 400-mL glass beakers with 350 mL of EC well water only. Solutions were renewed every 72 h, because preliminary work showed that this frequency was logistically feasible, did not result in fish mortality, and limited declines in EE2 concentrations to approximately one-third. The EC well water has the following characteristics: 0.20 mg/l total solids, 0.10 mg/l total dissolved solids, 0.001 mg/l total suspended solids, and 15.5 mg/l calcium. Water pH and temperature were measured twice monthly in the exposure beakers, generally during early morning or late afternoon; pH averaged 7.03; monthly mean water temperatures ranged from 24.22 to 24.13 °C (Supplemental Data, Table S1). During colder months, water heaters were placed in water surrounding the beakers to promote reproduction. Oxygen levels were not monitored, because these fish can handle low dissolved oxygen levels by gulping air from the air/water interface. When a fish reached sexual maturity, its age, sex, and standard length were documented (see “Time until sexual maturity” below for details on how sex and sexual maturity were determined). Because the sex of the two fish initially placed together in a beaker could not be determined until they were close to reaching sexual maturity, fish were exchanged as needed to have a male and a female fish in each beaker. If a beaker contained two fish of the same sex, one was replaced with a fish of the opposite sex from another replicate in the same treatment. Initially, we set up 70 beakers with two 0-6-day-old fish (of unknown sex) per exposure group to obtain the desired 30 male + female pairs of fish per group. Fish were fed as described above for the breeding stock. For females, size (as standard length) was measured once per month, at the point where they reached sexual maturity, and again monthly until the end of the experiment (8-month time point). Because male H. formosa grow very little once they become sexually mature, monthly length measurements were not taken once they reached sexual maturity with the exception of the 8-month time point. Fish body weights also were determined at the end of the experiment. Other variables quantified were time to sexual maturity, number of offspring per breeding pair (from sexual maturity to end of the experiment), number of broods, time between broods, number of offspring per brood, and offspring survival rate.
The first 15 offspring from EE2-exposed and control breeding pairs were exposed under the same conditions (including EE2 concentration) as their parents and reared to 16 weeks of age. Their survival was monitored biweekly starting with day 3. We also had planned to determine the time to sexual maturity and the sex ratio of the offspring. However, none of the EE2-exposed offspring survived long enough to reach sexual maturity.
Time Until Sexual Maturity
Time until sexual maturity was determined for the F0 fish. In female least killifish, sexual maturity is indicated by the appearance of a small black dot on the anal fin (Fraser and Renton 1940). Males are considered to be sexually mature once the third, fourth, and fifth anal rays have fused and elongated to form the gonopodium, which is used for sperm transfer (Constanz 1989; Rosen and Gordon 1953). Phenotypic sex was determined once fish approached sexual maturity (sex could not be determined in immature fish). Identification of the sex of individual fish that had not yet reached sexual maturity was based on the presence of a rounded anal fin in females and this fin becoming elongated in males.
Size, Condition Factor, and Growth Measurements
Standard length was determined from photos taken while a fish was lying on its side on a wet Plexiglas surface with a ruler. This procedure was done quickly (less than 10 s) to avoid permanent damage to the fish. Images were digitized using a Kodak Full High Definition 30X Easy Share Max digital photo camera. Standard length was measured from the tip of the snout to the caudal peduncle, using Image J Software (Abramoff et al. 2004) to optimize magnification for increased accuracy. Because H. formosa is sexually dimorphic, standard length was calculated separately for males and females. Fish wet weights were determined at the 8-month time point, after fish were euthanized. Preliminary experiments in our lab revealed that the small size of least killifish prevented us from obtaining precise weights of live fish. After euthanization, excess water was removed from the outer surface of the fish by gently blotting the body surface with a paper tissue just prior to weighing. Growth rates were assessed from the point of sexual maturity to eight months after birth and calculated from monthly measurements taken during that period.
The body condition factor was determined to assess both females’ and males’ condition and well-being (Nehemia et al. 2012). Condition factors were determined using the equation K = \(\frac{{{\text{weight}} \left( {\text{g}} \right)}}{{{\text{length}}^{3 } \left( {\text{cm}} \right)}}\), where K is condition factor (Versonnen et al. 2003), using the standard lengths and weights measured at the end of the experiment. For males exposed to 5 ng/L, one value was left out as a statistically significant outlier, although this did not affect the outcome of the statistical analysis.
Quantification of EE2 Levels
Concentrations of waterborne EE2 were verified using 150 mL of water from randomly selected beakers. Water samples (from solvent controls and exposure treatments) were collected before and just after water changes, because this represented the likely lower and higher limits of EE2 concentration experienced by the fish. Each 150-mL water sample was filtered with 0.2-μm pore-size filters before analysis and analyzed within 24 h after collection to keep microbial degradation to a minimum (Fonseca et al. 2013). Water samples were analyzed by using a Waters 510 (Waters Corporation) high-performance liquid chromatography system and measured against 17α-ethinylestradiol standards using reverse-phase chromatography with spectroscopic detection (Montgomery et al. 2012). Both of the EE2 standards and samples were concentrated by solid-phase extraction using C18-SD cartridges (3 M Empore) according to the manufacturer’s instructions. Standards and samples were subsequently resuspended in 200-µL mobile phase (40:60 water:methanol) and were manually injected and analyzed on a 3-µm particle size, 2 × 100 mm2 C18 column (Waters Corporation) using 40:60 water:methanol as the mobile phase with a flow rate of 1.5 mL/min. EE2 was not detected in the water of the solvent-control group (the detection limit was 0.5 ng/L).
Statistical Analyses
All statistical analyses used SAS Enterprise Guide 4.3 On Demand for Academics or JMP Versions 10.1 and 12.1 (SAS Institute). Before analyses, assumptions of normality were checked using the Shapiro–Wilk test (p > 0.05). Analysis of variance (ANOVA) was performed to compare the various endpoints among the three EE2 treatments. Where the overall ANOVA model was significant, pairwise comparisons (using Tukey–Kramer HSD) were used to assess which groups differed from each other. Survival of F1 fish was analyzed using a Kaplan–Meier survival analysis with log-rank tests.
Results
Exposure Concentrations of EE2
EE2 measurements in solutions with 5 ng/L nominal EE2 concentration ranged from 3.32 to 3.44 ng/L (average 3.39 ± 0.02) just before water changes and from 5.27 to 5.31 (and averaged 5.29 ± 0.005) just after water changes. EE2 measurements in solutions with 25 ng/L nominal EE2 concentration ranged from 17.39 to 17.66 ng/L (average 17.54 ± 0.04 just before water changes and from 25.33 to 25.51 (average 25.46 ± 0.02) just after water changes (Supplemental Data, Table S2).
Size, Growth, and Condition Factor (in F0)
Standard length was measured at sexual maturity and repeated monthly throughout the duration of the study (8 months) for both male and female fish. At sexual maturity, there was an overall significant effect of EE2 exposure on male standard lengths (F2,87 = 42.96, p < 0.0001). Male size in both EE2 exposure groups was larger than that of the control fish, whereas the size did not differ between the two EE2 exposure groups in pairwise comparisons (p = 0.2077; Fig. 1). Among the 8-month-old males, there was again an overall significant effect of EE2 exposure on standard length (F2,87 = 63.20, p < 0.0001). Pairwise comparisons once more showed that males from the two exposure groups were significantly larger than the control males, but males from the two exposure groups did not differ significantly in size (p = 0.3184; Fig. 2).
For females, there were no significant differences in standard length among treatments at sexual maturity (F2,87 = 1.43, p = 0.2449; Fig. 1), but these did differ for the 8-month-old females (F2,87 = 68.58, p < 0.0001). At that time point, females of both exposure groups were significantly smaller than control females, but there was no difference in size between females from the two exposure groups (p = 0.5626; Fig. 2).
Growth rates were determined for the period from sexual maturity to 8 months after birth. While there were statistically significant differences in overall growth rates among treatments for both sexes (F2,87 = 6.56, p < 0.0022 for males and F2,87 = 52.52, p < 0.0001 for females), specific patterns differed among the sexes. Pairwise comparisons showed that males exposed to 25 ng of EE2/L grew significantly faster than control males did (p = 0.0077), but no increase relative to the control fish was observed for the males exposed to 5 ng of EE2/L (p = 0.9942). However, the growth rate differed significantly among the males of the two EE2-exposed groups (p = 0.0056; Fig. 3). In contrast, females exposed to 5 and 25 ng/L grew significantly slower than was the case for the control females (p < 0.0001). There were, however, no statistically significant differences in the females’ growth rate between the two EE2-exposure groups (p = 0.7693; Fig. 3).
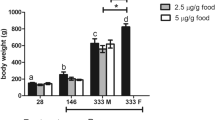
Condition factors were determined for the F0 fish at the end of the experiment. Male condition factors were significantly different among treatments overall (F2, 86 = 46.92, p < 0.0001) and differed significantly between specific treatments—decreasing as a function of EE2 exposure level (Fig. 4). While condition factors were generally higher for females than they were for males, they showed the same overall patterns. Condition factors differed significantly among treatments (F2,79 = 11.32, p < 0.0001) and were again highest for control fish and lowest for the fish exposed to 25 ng/L EE2 (Fig. 4).
Condition factor (K) of F0 generation male and female least killifish (H. formosa) exposed continuously from birth to control conditions, 5 ng/L EE2 or 25 ng/L EE2. Values are mean (± SE; n = 22–30) condition factor (10−2 g/cm3) at 8 months of age. Groups with the same letter did not differ significantly (p > 0.05) in a posteriori pairwise comparisons
Time to Sexual Maturity (in F0)
For both males and females, the time required to reach sexual maturity differed significantly among treatments (F2, 87 = 1278.08, p < 0.0001 and F2, 87 = 664.22, p < 0.0001 respectively; Fig. 5). Pairwise comparisons revealed significant differences among the three groups for both sexes. For males exposed to 5 ng/L EE2, the time needed to reach sexual maturity was increased by approximately 57% compared with the situation for control males, whereas the analogous figure for males exposed to 25 ng/L EE2 was a 90% increase. Meanwhile, the time needed to reach sexual maturity was increased by 56% and 90% (compared the situation for control females) for females exposed to 5 ng/L EE2 and those exposed to 25 ng/L EE2, respectively (Fig. 5).
Reproduction (in F0)
The total fecundity of breeding pairs of H. formosa differed significantly among treatments (F2, 87 = 6.30, p = 0.001; Fig. 6a). The fecundity of fish exposed to 5 ng EE2/L was reduced by approximately 50% relative to the situation for the control fish. While numerical values were reduced by another 25% for the fish in the 25 ng/L EE2 group, the difference between the two EE2 exposure groups was not statistically significant (t (58) = 1.5187, p = 0.1343). The lower fecundity of the EE2-exposed fish was at least partly due to fewer broods produced per female. The number of broods differed among treatments (F2,87 = 6.587, p = 0.0022; Fig. 6b): lower for the fish in the two EE2 treatments, but not significantly different between the latter treatments. There were no significant differences among treatments with respect to the brood interval (F2,87 = 0.777, p = 0.463; data not shown). While brood sizes did not differ statistically (F2, 119 = 1.567, p = 0.21), there was a tendency for brood sizes to be lower in fish exposed to 25 ng/L EE2 (Fig. 6c).
Reproductive success through eight months of age of F0 generation breeding pairs of least killifish (H. formosa) exposed continuously from birth, to control conditions, 5 ng/L EE2 or 25 ng/L EE2. Groups with the same letter did not differ significantly (p > 0.05) in a posteriori pairwise comparisons. a Total number of offspring per breeding pair (mean ± SE; n = 30). b Total number of broods per breeding pair (mean ± SE; n = 30). c Number of offspring per brood (mean ± SE; n = 30)
Survival (in F1)
Offspring survival was monitored from day 3 until week 16. There was a significant difference in offspring survival among treatments (χ2 = 80.4, p = 0.001), and survival was reduced for both EE2-exposure groups (Fig. 7). However, survival did not differ significantly among those two groups (χ2 = 0.223, p = 0.64). The EE2-exposed offspring did not survive long enough to reach sexual maturity, in contrast to the situation for the offspring of the control breeding pairs—where 87% of the offspring reached sexual maturity (with a 46:54 male:female sex ratio).
Survival (from birth to 16 weeks) of F1 generation H. formosa offspring (n = 15 per group). Both the F0 generation and these offspring were exposed continuously from birth to control conditions, 5 ng/L EE2 or 25 ng/L EE2. Groups with the same letter did not differ significantly (p > 0.05) in the survival analysis
Discussion
The present study revealed a variety of effects on growth, development, and reproduction in least killifish exposed continuously to nominal levels of 5 ng/L and 25 ng/L of the synthetic estrogen EE2, with actual concentrations fluctuating between 3.4 and 5.3 ng/L for the lower nominal level and between 17.4 and 25.5 for the higher nominal level. Ethinylestradiol has been detected in receiving waters from sewage treatment plants at concentrations ranging from 0.1 to 10 ng/L (Christiansen et al. 2002; Ternes et al. 1999) and in sewage treatment plant discharges at concentrations spanning the 10 to 142 ng/L range (Desbrow et al. 1998; Hemming et al. 2002; Kolpin et al. 2002; Larsson et al. 1999). When the presence of other natural estrogenic compounds, such as estrone (E1), 17β-estradiol (E2), and plant-derived phytoestrogens also is accounted for, U.S. surface waters receiving sewage treatment plant effluents may actually contain up to 300 ng/L of EE2 equivalents (Lazorchak et al. 2003). Those data illustrate that the range of concentrations used in present study is environmentally relevant for the exposure of fish.
While the present study’s results did demonstrate that concentrations falling within this range resulted in multiple effects, logistical limitations prevented us from having sufficient different exposure concentrations to do a thorough evaluation of dose–response relationships for those effects. Among the two different EE2 levels used, only in a few instances were effects more severe at 25 ng/L than at 5 ng/L; more typically, there was a lack of a significant difference among the two EE2 levels. An absence of typical dose–response relationships, with sometimes a hormesis-type response, is not unusual for EDCs—and also was observed in fathead minnows exposed to EE2 (Bergman et al. 2012). In general, hormones, such as EE2, produce effects by acting on specialized receptors, such as estrogen receptors, which may be present at such a limited abundance that effects do not increase above a relatively low EDC concentration. Moreover, environmental EDCs may add to the endogenous hormonal effects, such that low concentrations of EDCs can produce responses much stronger than would be expected on the basis of their concentration (Bergman et al. 2012). Furthermore, an increased abundance of hormones can cause a down-regulation of receptor binding (Bergman et al. 2012), also negating a typical dose–response relationship. A more thorough evaluation of the dose–response relationship and an assessment of the hormone-receptor interactions of EE2 in H. formosa is needed for more definitive insights in these issues.
Growth and overall health of H. formosa were affected by EE2 exposure, with effects on growth differing between males and females. In males, exposure to EE2 resulted in increased length at sexual maturity and an increased length at the end of the experiment; female length at sexual maturity was not affected, whereas it was reduced at the 8-month time point. These patterns are consistent with EE2 effects on growth after reaching sexual maturity differing between the sexes: positive in males and negative in females. In contrast, EE2 exposure resulted in a reduced condition factor in both sexes. The effects on size agree with those from previous studies. Kristensen et al. (2005) reported an increased standard length of male guppies (Poecilia reticulata), a live-bearing species of fish, exposed to 112 ng/L EE2. A reduction in females’ body length was reported in zebrafish exposed to 25 ng/L EE2 for 3 months (Van den Belt et al. 2003) and in fathead minnows exposed to 1 and 4 ng/L EE2 for 56 days post-hatch (Lange et al. 2001). While the body condition factor K is a good indicator of fish health (Blackwell et al. 2000), we are not aware of previous studies having included this variable in assessments of the effects of EE2 in fish. Because K (as weight/length3) is directly related to weight, a lower weight will result in a lower K. Various studies have reported lower body weights in female fish due to EE2 (Foran et al. 2002; Kristensen et al. 2005; Lange et al. 2001).
In the present study, EE2 exposure resulted in both males and females taking a longer time to become sexually mature and, once sexually mature, producing fewer offspring. The delay in reaching sexual maturity was substantial; pairs exposed to 5 and 25 ng/L EE2 took 48 and 76 days longer (respectively) to reach sexual maturity than was the case for control pairs. The reduction in the number of offspring produced by EE2-exposed pairs also was substantial; numbers were reduced by 50–75% compared with control pairs. Exposure to EE2 has been shown to reduce the numbers of eggs spawned, those hatched, or those reaching a particular developmental stage for oviparous fish (Foran et al. 2002; Kidd et al. 2007; Lange et al. 2001; Scholz and Gutzeit 2000; Schultz et al. 2003).
Finally, the continued EE2 exposure of offspring resulted in a strong impact on survival; none of the offspring survived longer than 8 weeks. These findings are consistent with those from a study by Nash et al. (2004) in which effects from exposure to EE2 continued into the second generation. The occurrence of increased effects of continued EE2 exposure in subsequent generations of oviparous fish species, resulting in negative impacts at the population level, also have been reported previously (Yan et al. 2012; Kidd et al. 2007).
Various mechanisms may have been responsible for the observed EE2 effects in the present study as well as for the interrelationships among effects; more research is needed to understand these fully. The increased size at sexual maturity among males may be due to the feminizing effect of EE2, because female least killifish are typically much larger than males. Similar to the findings of Lange et al. (2001), there was evidence of feminization in the EE2-exposed male least killifish (ova-testes were detected in preliminary histological analyses). Because male poeciliids grow mostly before reaching sexual maturity (Kolpin et al. 2002; Trexler et al. 1990), it is possible that the longer time taken for males to reach sexual maturity may have resulted in larger males at sexual maturity. However, this may be only part of the reason, because EE2-enhanced growth continued beyond the point of sexual maturity—at least for the fish exposed to 25 ng/L of EE2. For both sexes, the increase in time-to-sexual maturity resulted in a decrease in time remaining (within the 8-month experimental period) to produce offspring, and this could have contributed to a reduced offspring production by lowering the number of broods produced. While the observed reduction in offspring production therefore may appear to be an artifact caused by having a fixed experimental period, this scenario is actually comparable to the natural situation in most of this species range where the annual reproductive period is limited to approximately 6-8 months as well. The decrease in number of offspring may have been a consequence of a decrease in the number of mature eggs within the ovaries of exposed females. Preliminary evidence of this was obtained in an ongoing histopathological analysis of EE2-exposed least killifish. The reduction in offspring production also could have been due to the lower size and condition factor of the females; bigger poeciliid females tend to produce more offspring (Constanz 1989). While EE2-exposed males were larger, this increased body size may actually have had a negative consequence for reproduction. Because copulations in these fish are unsolicited, bigger males have a harder time positioning themselves in a female’s blind spot and copulate successfully. Effects on males may have reduced offspring production as a consequence of EE2 impact on sperm development (which appears to be the case based on the findings in an ongoing histopathological study) or impact on male reproductive behavior (Jackson et al. 2018). For a related fish, the guppy Poecilia reticulata, Kristensen et al. (2005) showed that males exposed to EE2 sired fewer offspring and had less copulation attempts (gonopodial thrusts) than control fish. It is unknown whether EE2 affects reproductive behavior of male least killifish. Finally, effects may have been due to the stress from the EE2 exposure, resulting in less energy allocated to growth, sexual development, or reproduction (Martin et al. 2009).
The findings of this study of the various effects that result from EE2 exposure point to the likelihood of ecological consequences. The ecological impact of estrogenic-endocrine disruptors is typically mediated by effects on reproduction (Lange et al. 2001). The present study’s observations of delayed sexual maturity, smaller female body size (a variable known to be inversely related to fecundity), and reduced offspring production (in part due to fewer broods produced) all point to effects on reproduction being crucial here as well. Furthermore, the finding that EE2 not only affected offspring production but also survival in the next generation indicates the potential for impact at the population level. While this is being evaluated in an accompanying study, the potential for negative effects was clearly demonstrated for an oviparous fish species (the fathead minnow) in a whole lake study conducted at the Experimental Lakes Area in Ontario, Canada (Kidd et al. 2007). That study showed that the exposure resulted in reproductive failure and the collapse of the fathead minnow population in an EE2-dosed lake.
In long-term, EE2-exposure studies with the oviparous fish species roach (Rutilus rutilus), fathead minnow (Pimephales promelas), zebrafish (Danio rerio), and medaka (Oryzias latipes), the observed effects included the presence of males with female-like gonad morphology or fully sex-reversed males, populations become female-only, decreases in body size for both sexes, and decreases in fertilization and hatching success (Faulk et al. 1999; Rolland 2000; Sumpter 2005; Yan et al. 2012). The present study did not find any evidence of sex-reversed males, and the effect on male body size was opposite to the effect observed in oviparous fish. The larger male body size also was observed in the viviparous guppy (Kristensen et al. 2005), indicating that this response in males may differ fundamentally between viviparous and oviparous fish. The present study’s observed effects on time-to-sexual maturity, reproduction, and offspring survival were similar in direction to effects observed in oviparous fish but appeared to be more severe and to occur more rapidly in least killifish. These results indicate that while some specific effects of EE2 exposure may differ between viviparous and oviparous fish, the differences in environment and nutritional supply during development and the differences in the specific roles of estrogen do not appear to result in the matrotrophic viviparous least killifish being less vulnerable to effects of estrogenic endocrine disruptors, such as EE2. The severe effects on reproduction and offspring survival observed in the present study also indicate that having a K-strategy may make a fish species more vulnerable to endocrine disruptors than is the case for fish species with an r-strategy. More studies are needed to understand fully the influences of life history and reproductive strategy on the long-term environmental effects of EDCs.
Conclusions
This study showed that in least killifish continuous EE2 exposure affected growth and sexual development of both males and females, strongly reduced reproduction, and greatly reduced survival in the second generation. These results indicate that live-bearing fish with matrotrophic development and a K-strategy with respect to reproductive investment appear to be at least as vulnerable to ecological impacts from estrogenic-endocrine disruptors, such as EE2, than one would expect on the basis of the many studies conducted with egg-laying fish species.
Data Availability
Data pertaining to this manuscript are available, upon request, from the lead author (LMJ).
References
Abramoff M, Magalhaes P, Ram S (2004) Image processing with image. J Biophotonics 11:36–42
Al-Ansari A, Saleem A, Kimpe L, Sherry J, McMaster M, Trudeau V, Blais J (2010) Bioaccumulation of the pharmaceutical 17α-ethinylestradiol in shorthead redhorse suckers (Moxostoma macrolepidotum) from the St. Clair River, Canada. Environ Pollut 158:2566–2571
Balch G, Mackenzie C, Metcalfe C (2004) Alterations to gonadal development and reproductive success in Japanese medaka (Oryzias latipes) exposed to 17α-ethinylestradiol. Environ Toxicol Chem 23:782–791
Barel-Cohen K, Shore L, Shemesh M, Wenzel A, Mueller J, Kronfeld-Schor N (2006) Monitoring of natural and synthetic hormones in a polluted river. J Environ Manag 78:16–23
Belfroid A, Van der Horst A, Vethaak A, Schafer A, Rijs G, Wegener J, Cofino W (1999) Analysis and occurrence of estrogenic hormones and their glucuronides in surface water and waste water in The Netherlands. Sci Total Environ 225:101–108
Belk M, Lydeard C (1994) Effect of Gambusia holbrooki on a similar-sized, syntopic Poeciliid, Heterandria formosa: competitor or predator? Copeia 1994:296–302
Bergman Å, Heindel J, Jobling S, Kidd K, Zoeller R (2012) State-of-the-science of endocrine disrupting chemicals, 2012: an assessment of the state of the science of endocrine disruptors prepared by a group of experts for the United Nations Environment Programme and World Health Organization. Toxicol Lett 211:S3
Bhandari R, Saal F, Tillitt D (2015) Transgenerational effects from early developmental exposures to bisphenol A or 17α-ethinylestradiol in medaka, Oryzias latipes. Sci Rep. https://doi.org/10.1038/srep09303
Bizarro C, Ros O, Vallejo A, Prieto A, Etxebarria N, Cajaraville M, Ortiz-Zarragoitia M (2014) Intersex condition and molecular markers of endocrine disruption in relation with burdens of emerging pollutants in thicklip grey mullets (Chelon labrosus) from Basque estuaries (South-East Bay of Biscay). Mar Environ Res 96:19–28
Blackwell B, Brown M, Willis D (2000) Relative weight (Wr) status and current use in Fisheries assessment and management. Rev Fish Sci 8:1–44
Buchberger W (2007) Novel analytical procedures for screening of drug residues in water, waste water, sediment and sludge. Anal Chim Acta 593:129–139
Cargouet M, Perdiz D, Mouatassim-Souali A, Tamisier-Karolak S, Levi Y (2004) Assessment of river contamination by estrogenic compounds in Paris area (France). Sci Total Environ 324:55–66
Chen C, When T, Wang G, Cheng H, Lin Y, Lien G (2010) High estrogen concentrations in receiving river discharg from a concentrated livestock feedlot. Sci Total Environ 408:3223–3230
Cheong R, Henrich S, Farr J, Travis J (1984) Variation in fecundity and its relationship to body size in a population of the least killifish, Heterandria formosa (Pisces: Poeciliidae). Copeia 3:720–726
Christiansen L, Winther-Nelsen M, Helweg C (2002) Feminization of fish: the effect of estrogenic compounds and their fate in sewage treatment plants and nature (Environmental Project No. 720-2002). Danish Environmental Protection Agency, København, Denmark
Clouzot L, Marrot B, Doumenq P, Roce N (2008) 17 alpha-ethinylestradiol: an endocrine disrupter of great concern. Analytical methods and removal processes applied to water purification. A review. Environ Progr Sustain Energy 27:383–396
Constanz G (1989) Reproductive biology of the poeciliid fishes. In: Meffe GK, Snelson FF Jr (eds) Englewood Cliffs. Prentice Hall, NJ
Desbrow C, Routledge E, Brighty G, Sumpter J, Waldock M (1998) Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environ Sci Technol 32:1549–1557
Diaz-Cruz S, Barcelo’ D (2004) Occurrence and analysis of selected pharmaceuticals and metabolites as contaminants present in wastewaters, sludge and sediments. Handb Environ Chem 5:227–260
Duong C et al (2010) Estrogenic chemicals and estrogenicity in river waters of South Korea and seven Asian countries. Chemosphere 78:286–293
Dziewecyznski T, Hebert O (2013) The effects of short-term exposure to an endocrine disrupter on behavioural consistency in male juvenile and adult Siamese fishting fish. Arch Environ Contam Toxicol 64:316–326
Faulk C, Fuiman L, Thomas P (1999) Parental exposure to ortho, paradichlorodiphenyltrichloroethane impairs survival skills of Atlantic croaker (Micropogonias undulatus) larvae. Environ Toxicol Chem 18:254–262
Fonseca A, Cardoso M, Esteves V (2013) Determination of estrogens in raw and treated wastewater by high-performance liquid chromatograpy: ultraviolet detection. J Environ Anal Toxicol 4:203
Foran C, Peterson B, Benson W (2002) Transgenerational and developmental exposure of Japanese medaka (Oryzias latipes) to ethinylestradiol results in endocrine and reproductive differences in the response to ethinylestradiol as adults. J Toxicol Sci 68:389–402
Fraser E, Renton R (1940) Observation on the breeding and development of the viviparous fish Heterandria formosa. Q J Microsc Sci 81:479–520
Gadd J, Tremblay L, Northcott G (2010) Steroid estrogens, conjugated estrogens and estrogenic activity in farm dairy shed effluents. Environ Pollut 158:730–736
Gennotte V et al (2015) Brief exposure of embryos to steroids or aromatase inhibitor induces sex reversal in Nile tilapia (Oreochromis niloticus). J Exp Zool 323A:31–38
Gibson R, Smith M, Spary C, Tyler C, Hill A (2005) Mixtures of estrogenic contaminates in the bile of fish exposed to wastewater treatment works effluents. Environ Sci Technol 39:2461–2471
Hardman J, Limbird L, Molinoff P, Ruddon R, Goodman A (1996) Goodman and Gilman’s: the pharmacological basis of therapeutics. Toxicol Lett 86:1–55
Hemming J, Turner P, Brooks B, Waller W, LaPoint T (2002) Assessment of toxicity reduction in wastewater effluent flowing through a treatment wetland using Pimephales promelas, Ceriodaphnia dubia, and Vibrio fischeri. Arch Environ Contam Toxicol 42:9–16
Jackson L, Felgenhauer B, Klerks P (2018) Feminization, altered gonadal development, and liver damage in least killifish (Heterandria formosa) exposed to sublethal concentrations of 17α-ethinylestradiol. Ecotox Environ Safe 170:331–337
Johnson A (2010) Natural variations in flow are critical in determining concentrations fo point source contaminants in rivers; as estrogen example. Environ Sci Technol 44:7865–7870
Johnson A, Sumpter P (2014) Putting pharmaceuticals into the wider context of challenges to fish populations in rivers. Phil Trans R Soc B. https://doi.org/10.1098/rstb.2013.0581
Jurgens M, Holthaus K, Johnson A, Smith J (2002) The potential for estradiol and ethynylestradiol degradation in English rivers. EnvironToxicol Chem 21:480–488
Kah O, Dufour S (2011) Conserved and divergent features of reproductive neuroendocrinology in teleost fishes. In: Norris D, Lopez K (eds) Hormones and reproduction of vertebrates. Academic Press, London, pp 15–42
Kidd K, Blanchfield P, Mills K, Palace V, Evans R, Lazorchak J, Flick R (2007) Collapse of a fish population after exposure to a synthetic estrogen. PNAS 104:8897–8901
Kitamura S, Ogata H, Takashima F (1994) Activities of F-type prostaglandins as releaser sex pheromones in cobitide loach. Misgurnus anguillicaudatus Comp Biochem Physiol A 107:161–169
Kolpin D, Furlong E, Meyer M, Thurman E, Zaugg S, Barber L, Buxton H (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36:1202–1211
Kostich M, Flick R, Martinson J (2013) Comparing predicted estrogen concentrations with measurements in U.S. waters. Environ Pollut 178:271–277
Kristensen T, Baatrup E, Bayley M (2005) 17α-ethinylestradiol reduces the competitive reproductive fitness of the male guppy (Poecilia reticulate). Biol Reprod 72:150–156
Kunz P, Kienle C, Carere M, Homazava N, Kase R (2015) In vitro bioassays to screen for endocrine active pharmaceuticals in surface and waste waters. JPBA 106:107–115
Lange R et al (2001) Effects of the synthetic estrogen 17α-ethinylestradiol on the life-cycle of the fathead minnow (Pimephales promelas). Environ Toxicol Chem 20:1216–1227
Lange A, Paull G, Hamilton P, Iguchi T, Tyler C (2011) Implications of persistent exposure to treated wastewater effluent for breeding in wild roach (Rutilus rutilus) populations. Environ Sci Technol 45:1673–1679
Langer R (2009) Efficacy, safety, and tolerability of low-dose hormone therapy in managing menopausal symptoms. J Am Board Fam Med 22:563–573
Larsson D, Adolfsson-Erici M, Parkkonen J, Petterson M, Berg A, Olsson P, Forlin L (1999) Ethynylestradiol: an undesired fish contraceptive. Aquat Toxicol 454:91–97
Lavelle C, Sorensen P (2011) Behavioral responses of adult male and female fathead minnows to a model estrogenic effluent and its effects on exposure regime and reproductive success. Aquat Toxicol 101:521–528
Lazorchak J, McCormick F, Henry T, Herlihy A (2003) Contamination of fish in streams of the Mid-Atlantic Region: an approach to regional indicator selection and wildlife assessment. Environ Toxicol Chem 22:545–553
Lei B, Huang S, Zhou Y, Wang D, Wang Z (2009) Levels of six estrogens in water and sediment from three rivers in Tianjin area, China. Chemosphere 76:36–42
Liu J, Wang R, Huang B, Lin C, Zhou J, Pan X (2012a) Biological effects and bioaccumulation of steroidal and phenolic endocrine disrupting chemicals in high-back crucian carp exposed to wastewater treatment plant effluents. Environ Pollut 162:325–331
Liu S, Ying G, Zhou L, Zhang R, Chen Z, Lai H (2012b) Steroids in typical swine farm and their release into the environment. Water Res 45:3754–3768
Martin S, Hitch A, Purcell K, Klerks P, Leberg P (2009) Life history variation along a salinity gradient in coastal marshes. Aquat Biol 8:15–28
Montgomery T, Brown A, Gendelman H, Ota M, Clottfelter E (2012) Exposure to 17α-ethinylestradiol decreases motility and ATP in sperm of male fighting fish Betta splendens. Environ Toxicol 29:243–252
Nash J, Van der Ven L, Brion F, Maack G, Stahlschmidt-Allner P, Tyler C (2004) Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environ Health Perspect 112:1725–1733
Nehemia A, Maganira J, Rumisha C (2012) Length-weight relationship and condition factor of talapia species grown in marine and fresh water ponds. Agric Biol J North Am 3:117–124
Nelson E, Do H, Lewis R, Carr S (2011) Diurnal variability of pharmaceutical, personal care product, estrogen and alkylphenol concentrations in effluent from a tertiary wastewater treatment facility. Environ Sci Technol 45:1228–1234
Pojana G, Gomiero A, Jonkers N, Marcomini A (2007) Natural and synthetic endocrine disrupting compounds (EDCs) in water, sediment and biota of a coastal lagoon. Environ Int 33:929–936
Reznick D, Callahan H, Llauredo R (1996) Maternal effects on offspring quality in poeciliid fishes. Am Zool 36:147–156
Rolland R (2000) Ecoepidemiology of the effects of pollution on reproduction and survival of early life stages in teleosts. Fish Fish 1:41–72
Rosen D, Bailey R (1963) The poeciliid fishes (Cyprinodontiformes), their structure, zoo-geography, and systematics. B Am Mus Nat Hist 126:1–176
Rosen D, Gordon M (1953) Functional anatomy and evolution of male genitalia in poeciliid fishes. Zoologica 38:1–47
Saaristo M, Craft J, Lehtonen K, Lindstrom K (2009) Sand goby (Pomatoschistus minutus) males exposed to endocrine disrupting chemical fail in nest and mate competition. Horm Behav 56:315–321
Salla R et al (2016) Impact of an environmental relevant concentration of 17α-ethinylestradiol on the cardiac function of bullfrog tadpoles. Chemosphere 144:1862–1868
Sarat M, Rambabu C (2012) A validated simultaneous RP-HPLC method for determination of desogestrel and ethinyl estradiol tablets. Int J Pharm Pharm Sci 4:115–119
Scholz S, Gutzeit H (2000) 17-a-ethynylestradiol affects reproduction, sexual differentiation and aromatase gene expression of the medaka (Oryzias latipes). Aquat Toxicol 50:363–373
Schultz I, Skillman A, Nicolas J, Cyr D, Nagler J (2003) Short-term exposure to 17a-ethynylestradiol decreases the fertility of sexually maturing male rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 22:1272–1280
Schwindt A, Winkelman D, Keteles K, Murphy M, Vajda A (2014) An environmental oestrogen disrupts fish population dynamics through direct and transgenerational effects on survival and fecundity. J Appl Ecol 51:582–591
Scrimshaw N (1944) Embryonic growth in the viviparous poeciliid, Heterandria formosa. Biol Bull 87:37–51
Segner H, Navas J, Schafers C, Wenzel A (2003) Potencies of estrogenic compounds in in vitro screening assays and in life cycle tests with zebrafish in vivo. Ecotox Environ Safe 54:315–322
Shifren J, Schiff I (2010) Role of hormone therapy in the management of menopause. Obstet Gynecol 115:839–855
Silva P et al (2012) Testing the effects of ethinylestradiol and of an environmentally relevant mixture of xenoestrogens as found in the Douro River (Portugal) on the maturatioin of fish gonads: a stereological study using the zebrafish (Danio rerio) as model. Aquat Toxicol 124–125:1–10
Snyder S, Keith T, Verbrugge D, Snyder E, Gross T, Kannan K, Giesy J (1999) Analytical methods for detection of selected estrogenic compounds in aqueous mixtures. Environ Sci Technol 33:2814–2820
Sole’ M, Raldua D, Piferrer F, Barcelo’ D, Porte C (2003) Feminization of wild carp, Cyprinus carpio, in a polluted environment: plasma steroid hormones, gonadal morphology and xenobiotic metabolizing system. Comp Biochem Phys C 136:145–156
Sorensen P, Goetz F (1993) Pheromonal and reproductive function of F-prostaglandins and their metabolites in teleost fish. J Lipid Mediators 6:385–393
Stacey N, Peter R (1979) Central action of prostaglandins in spawning behavior of female goldfish. Physiol Behav 22:1191–1196
Sumpter J (2005) Endocrine disrupters in the aquatic environment: an overview. Acta Hydroch Hydrob 33:9–16
Sun L, Yong W, Chu X, Lin J (2009) Simultaneous determination fo 15 steroidal oral contraceptives in water using splid-phase disk extraction followed by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1216:5416–5423
Tamschick S et al (2016) Sex reversal assessments reveal different vulnerability to endocrine disruption between deeply diverged anuran lineages. Sci Reg. https://doi.org/10.1038/srep23825
Ternes T, Stumpf M, Mueller J, Haberer K, Wilken R, Servos M (1999) Behavior and occurrence of estrogens in municipal sewage treatment plants-I. Investigations in Germany, Canada and Brazil. Sci Total Environ 225:81–90
Teta C, Naik Y (2016) Vitellogenin induction and reduced fecundity in zebrafish exposed to effluents from the city of Bulawayo, Zimbabwe. Chemosphere 167:282–290
Travis J, Farr J, Henrich S, Cheong R (1987) Testing theories of clutch overlap with the reproductive ecology of Heterandria formosa. Ecology 68:611–623
Trexler J, Travis J, Trexler M (1990) Phenotypic plasticity in the sailfin molly, Poecilia latipinna (Pisces: Poeciliidae). II. Laboratory experiment. Evolution 44:157–167
Trudeau V, Metcalfe C, Mimeault C, Moon T (2005) Pharmaceuticals in the environment: drugged fish? Biochem Mol Biol Fishes 6:475–493
Turner C (1937) Reproductive cycles and superfetation in poeciliid fishes. Biol Bull 72:145–164
Van Aerle R, Nolanusan M, Jobling S, Christiansen L, Sumpter J, Tyler C (2001) Sexual disruption in a second species of wild cyprinid fish (the gudgeon, Gobio gobio) in United Kingdom freshwaters. Environ Toxicol Chem 20:2841–2847
Van den Belt K, Verheyen R, Witters H (2003) Effects of 17a-ethinylestradiol in a partial life-cycle test with zebrafish (Danio rerio): effects on growth, gonads and female reproductive success. Sci Total Environ 309:127–137
Versonnen B, Arijs K, Verslycke T, Lema W, Janssen C (2003) In vitro and in vivo estrogenicity and toxicity of o-, m-, and p-dichlorobenzene. Environ Toxicol Chem 22:329–335
Vieno N, Harkki H, Tuhkanen T, Kronberg L (2007) Occurrence of pharmaceuticals in river water and their elimination in a pilot-scale drinking water treatment plant. Environ Sci Technol 41:5077–5084
Wang L et al (2011) Assessing estrogenic activity in surface water and sediment of the Liao River systems in northeast China using combined chemical and biological tools. Environ Pollut 159:148–156
Williams R (2005) Human pharmaceuticals: assessing the impacts on aquatic ecosystems. Alan Press/ACG Publishing, Lawrence
World Health Organization (WHO) (2012) State of the science of endocrine disrupting chemicals- 2012. World Health Organization, International Programme on Chemical Safety, Geneva
Xu J, Wu L, Chen W, Jiang P, Chang A (2009) Pharmaceuticals and personal care products (PPCPs) and endocrine disrupting compounds (EDCs) in runoff from a potato field irrigated with treated wastewater in Southern California. J Health Sci 55:306–310
Yan Z, Lu G, Liu J, Jin S (2012) An integrated assessment of estrogenic contamination and feminization risk in fish in Taihu Lake, China. Ecotox Environ Safe 84:334–340
Yang Y, Gray J, Furlong E, Davis J, ReVello R, Borch T (2012) Steroid hormone runofff from agricultural test plots applied with municipal biosolids. Environ Sci Technol 45:2746–2754
Ying G, Rai K, Ying-Jun R (2002) Occurrence and fate of hormone steroids in the environment. Environ Int 28:545–551
Ying G, Kookana R, Kumar A, Mortimer M (2009) Occurrence and implications of estrogens and xenoestrogens in sewage effluents and receiving waters from South East Queensland. Sci Total Environ 407:5147–5155
Zhang Z, Feng Y, Gao P, Wang C, Ren N (2011) Occurrence and removal efficiencies of eight EDCs and estrogenicity in a STP. J Environ Monit 13:1366–1373
Zou Y, Zhang K, Zhou S (2013) Determination of estrogenic steroids and microbial and photochemical degradation of 17α-ethinylestradiol (EE2) in lake surface water: a case study. Environ Sci Process Impacts 15:1529–1535
Acknowledgements
The authors greatly appreciate the editorial comments from S. Duke-Sylvester, B. Felgenhauer, P. Leberg, and J. McLachlan, and the logistical support provided by J. Adeyemi, S. Volt, J. Hill, E. Blankson, A. Cazan, A. Oguma, A. Kascak, A. Pant, Q. Jackson, L. Carrier, and the staff of the UL Lafayette Ecology Center.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jackson, L.M., Klerks, P.L. Impact of Long-Term Exposure to 17α-Ethinylestradiol in the Live-Bearing Fish Heterandria formosa. Arch Environ Contam Toxicol 77, 51–61 (2019). https://doi.org/10.1007/s00244-019-00600-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-019-00600-5