Abstract
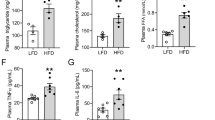
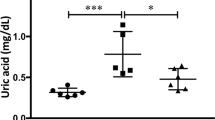
Specific relationships among reactive oxygen species, activation pathways, and inflammatory mechanisms involved in kidney injury were assessed in a combined model of obesity and hyperoxaluria. Male Wistar rats were divided into four groups: Control, HFD (high fat diet), OX (0.75% ethylene glycol), and HFD + OX (combined model) Changes in basal O2− levels were evaluated by chemiluminescence in renal interlobar arteries and renal cortex. Furthermore, the effect of different inhibitors on NADPH-stimulated O2− generation was assessed in renal cortex. Oxidative stress sources, and local inflammatory mediators, were also determined, in parallel, by RT-PCR, and correlated with measures of renal function, urinary biochemistry, and renal structure. Rats from the HFD group developed overweight without lipid profile alteration. Tubular deposits of crystals were seen in OX and severely enhanced in HFD + OX groups along with a significantly higher impairment of renal function. Basal oxidative stress was increased in renal cortex of OX rats and in renal arteries of HFD rats, while animals from the combined HFD + OX group exhibited the highest levels of oxidative stress in renal cortex, derived from xanthine oxidase and COX-2. NADPH oxidase-dependent O2− generation was elevated in renal cortex of the OX group and markedly enhanced in the HFD + OX rats, and associated to an up-regulation of Nox1 and a down-regulation of Nox4 expression. High levels of oxidative stress in the kidney, of OX and HFD + OX groups were also associated to an inflammatory response mediated by an elevation of TNFα, COX-2, NFκB1 MCP-1, and OPN. Oxidative stress is a key pathogenic factor in renal disease associated to hyperoxaluria and a common link underlying the exacerbated inflammatory response and kidney injury found under conditions of both obesity and hyperoxaluria. Nox1 pathway must be considered as a potential therapeutic target.








Similar content being viewed by others
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CaOx:
-
Calcium oxalate
- COX:
-
Cyclooxygenase
- CRD:
-
Chronic renal disease
- EG:
-
Ethylene glycol
- HFD:
-
High fat diet
- MCP:
-
Monocyte chemoattractant protein
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NO:
-
Nitric oxide
- Nox:
-
NADPH oxidase enzymes
- O2− :
-
Superoxide
- OPN:
-
Osteopontin
- ROS:
-
Reactive oxygen species
References
Scales CD, Smith AC, Hanley JM, Saigal CS, Urologic DIAP (2012) Prevalence of kidney stones in the United States. Eur Urol 62:160–165
Tungsanga K, Sriboonlue P, Futrakul P, Yachantha C, Tosukhowong P (2005) Renal tubular cell damage and oxidative stress in renal stone patients and the effect of potassium citrate treatment. Urol Res 33:65–69
Touyz RM, Briones AM, Sedeek M, Burger D, Montezano AC (2011) Nox isoforms and reactive oxygen species in vascular health. Mol Interv 11:27–35
Zuo J, Khan A, Glenton PA, Khan SR (2011) Effect of NADPH oxidase inhibition on the expression of kidney injury molecule and calcium oxalate crystal deposition in hydroxy-l-proline-induced hyperoxaluria in the male Sprague–Dawley rats. Nephrol Dial Transpl 26:1785–1796
Joshi S, Peck AB, Khan SR (2013) NADPH oxidase as a therapeutic target for oxalate induced injury in kidneys. Oxid Med Cell Longev 2013:462361
Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ (2008) Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int 73:19–33
Jonassen JA, Kohjimoto Y, Scheid CR, Schmidt M (2005) Oxalate toxicity in renal cells. Urol Res 33:329–339
Joshi S, Wang W, Peck AB, Khan SR (2015) Activation of the NLRP3 inflammasome in association with calcium oxalate crystal induced reactive oxygen species in kidneys. J Urol 193:1684–1691
Sáenz-Medina J, Jorge E, Corbacho C, Santos M, Sánchez A, Soblechero P et al (2018) Metabolic syndrome contributes to renal injury mediated by hyperoxaluria in a murine model of nephrolithiasis. Urolithiasis 46:179–186
Muñoz M, López-Oliva ME, Pinilla E et al (2017) CYP epoxygenase-derived H. Free Radic Biol Med 106:168–183
Khan SR (2005) Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res 33:349–357
Ebenezer PJ, Mariappan N, Elks CM, Haque M, Francis J (2009) Diet-induced renal changes in Zucker rats are ameliorated by the superoxide dismutase mimetic TEMPOL. Obesity (Silver Spring) 17:1994–2002
Munoz M, Sanchez A, Pilar Martinez M et al (2015) COX-2 is involved in vascular oxidative stress and endothelial dysfunction of renal interlobar arteries from obese Zucker rats. Free Radic Biol Med 84:77–90
Elmarakby AA, Imig JD (2010) Obesity is the major contributor to vascular dysfunction and inflammation in high-fat diet hypertensive rats. Clin Sci (Lond) 118:291–301
Wong WT, Wong SL, Tian XY, Huang Y (2010) Endothelial dysfunction: the common consequence in diabetes and hypertension. J Cardiovasc Pharmacol 55:300–307
Feig DI, Kang DH, Johnson RJ (2008) Uric acid and cardiovascular risk. N Engl J Med 359:1811–1821
Eleftheriadis T, Pissas G, Antoniadi G, Liakopoulos V, Stefanidis I (2018) Allopurinol protects human glomerular endothelial cells from high glucose-induced reactive oxygen species generation, p53 overexpression and endothelial dysfunction. Int Urol Nephrol 50:179–186
Khan SR, Joshi S, Wang W, Peck AB (2014) Regulation of macromolecular modulators of urinary stone formation by reactive oxygen species: transcriptional study in an animal model of hyperoxaluria. Am J Physiol Renal Physiol 306:F1285–F1295
Sedeek M, Nasrallah R, Touyz RM, Hébert RL (2013) NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J Am Soc Nephrol 24:1512–1518
Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS (2016) Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal 25:119–146
Sharma M, Sud A, Kaur T, Tandon C, Singla SK (2016) N-acetylcysteine with apocynin prevents hyperoxaluria-induced mitochondrial protein perturbations in nephrolithiasis. Free Radic Res 50:1032–1044
Muñoz M, Martínez MP, López-Oliva ME et al (2018) Hydrogen peroxide derived from NADPH oxidase 4- and 2 contributes to the endothelium-dependent vasodilatation of intrarenal arteries. Redox Biol 19:92–104
Chuang YH, Chuang WL, Huang SP, Liu CK, Huang CH (2009) Inhibition of nuclear factor-kappa B (NF-kappaB) activation attenuates ureteric damage in obstructive uropathy. Pharmacol Res 60:347–357
Taguchi K, Okada A, Hamamoto S et al (2015) Proinflammatory and metabolic changes facilitate renal crystal deposition in an obese mouse model of metabolic syndrome. J Urol 194:1787–1796
Szeto HH, Liu S, Soong Y, Alam N, Prusky GT, Seshan SV (2016) Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int 90:997–1011
Khan SR, Johnson JM, Peck AB, Cornelius JG, Glenton PA (2002) Expression of osteopontin in rat kidneys: induction during ethylene glycol induced calcium oxalate nephrolithiasis. J Urol 168:1173–1181
Merchant ML, Cummins TD, Wilkey DW et al (2008) Proteomic analysis of renal calculi indicates an important role for inflammatory processes in calcium stone formation. Am J Physiol Renal Physiol 295:F1254–F1258
Boonla C, Hunapathed C, Bovornpadungkitti S et al (2008) Messenger RNA expression of monocyte chemoattractant protein-1 and interleukin-6 in stone-containing kidneys. BJU Int 101:1170–1177
Funding
Spanish Urological Foundation. The Spanish Urological Association funded this study. AEU Research Grant 2017 and Grant SAF2016-77526-R from the Spanish Ministry of Economy and Competitivity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sáenz-Medina, J., Muñoz, M., Sanchez, A. et al. Nox1-derived oxidative stress as a common pathogenic link between obesity and hyperoxaluria-related kidney injury . Urolithiasis 48, 481–492 (2020). https://doi.org/10.1007/s00240-019-01170-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-019-01170-w