Abstract
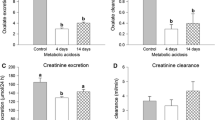
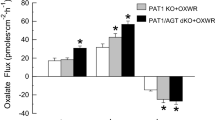
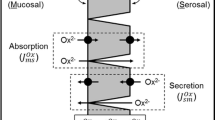
In rats, we recently showed how a chronic metabolic acidosis simultaneously reduced urinary oxalate excretion and promoted oxalate secretion by the distal colon leading to the proposition that acid–base disturbances may trigger changes to renal and intestinal oxalate handling. The present study sought to reproduce and extend these observations using the mouse model, where the availability of targeted gene knockouts (KOs) would offer future opportunities to reveal some of the underlying transporters and mechanisms involved. Mice were provided with a sustained load of acid (NH4Cl), base (NaHCO3) or the carbonic anhydrase inhibitor acetazolamide (ATZ) for 7 days after which time the impacts on urinary oxalate excretion and its transport by the intestine were evaluated. Mice consuming NH4Cl developed a metabolic acidosis but urinary oxalate was only reduced 46% and not statistically different from the control group, while provision of NaHCO3 provoked a significant 2.6-fold increase in oxalate excretion. For mice receiving ATZ, the rate of urinary oxalate excretion did not change significantly. Critically, none of these treatments altered the fluxes of oxalate (or chloride) across the distal ileum, cecum or distal colon. Hence, we were unable to produce the same effects of a metabolic acidosis in mice that we had previously found in rats, failing to find any evidence of the ‘gut-kidney axis’ influencing oxalate handling in response to various acid–base challenges. Despite the potential advantages offered by KO mice, this model species is not suitable for exploring how acid–base status regulates oxalate handling between the kidney and intestine.




Similar content being viewed by others
References
Hatch M, Freel RW (2008) The roles and mechanisms of intestinal oxalate transport in oxalate homeostasis. Semin Nephrol 28(2):143–151. https://doi.org/10.1016/j.semnephrol.2008.01.007
Whittamore JM, Hatch M (2017) The role of intestinal oxalate transport in hyperoxaluria and the formation of kidney stones in animals and man. Urolithiasis 45(1):89–108. https://doi.org/10.1007/s00240-016-0952-z
Robijn S, Hoppe B, Vervaet BA, D’Haese PC, Verhulst A (2011) Hyperoxaluria: a gut-kidney axis? Kidney Int 80(11):1146–1158. https://doi.org/10.1038/ki.2011.287
Costello JF, Smith M, Stolarski C, Sadovnic MJ (1992) Extrarenal clearance of oxalate increases with progression of renal-failure in the rat. J Am Soc Nephrol 3(5):1098–1104
Hatch M, Freel RW, Vaziri ND (1994) Intestinal excretion of oxalate in chronic-renal-failure. J Am Soc Nephrol 5(6):1339–1343
Hatch M, Freel RW (2003) Angiotensin II involvement in adaptive enteric oxalate excretion in rats with chronic renal failure induced by hyperoxaluria. Urol Res 31(6):426–432. https://doi.org/10.1007/s00240-003-0367-5
Hatch M, Cornelius J, Allison M, Sidhu H, Peck A, Freel RW (2006) Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int 69(4):691–698. https://doi.org/10.1038/sj.ki.5000162
Hatch M, Gjymishka A, Salido EC, Allison MJ, Freel RW (2011) Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with Oxalobacter. Am J Physiol Gastrointest Liver Physiol 300(3):G461–G469. https://doi.org/10.1152/ajpgi.00434.2010
Hatch M, Freel RW (2013) A human strain of Oxalobacter (HC-1) promotes enteric oxalate secretion in the small intestine of mice and reduces urinary oxalate excretion. Urolithiasis 41(5):379–384. https://doi.org/10.1007/s00240-013-0601-8
Whittamore JM, Hatch M (2015) Chronic metabolic acidosis reduces urinary oxalate excretion and promotes intestinal oxalate secretion in the rat. Urolithiasis 43(6):489–499. https://doi.org/10.1007/s00240-015-0801-5
Charney AN, Goldfarb DS, Dagher PC (1995) Metabolic disorders associated with gastrointestinal disease. In: Arieff AI, DeFronzo RA (eds) Fluid, electrolyte, and acid–base disorders, 2nd edn. Churchill Livingstone, New York, pp 813–836
Gennari FJ, Weise WJ (2008) Acid–base disturbances in gastrointestinal disease. Clin J Am Soc Nephrol 3(6):1861–1868. https://doi.org/10.2215/cjn.02450508
Charney AN, Feldman GM (1984) Systemic acid–base-disorders and intestinal electrolyte transport. Am J Physiol Gastrointest Liver Physiol 247(1):G1–G12
Charney AN, Dagher PC (1996) Acid–base effects on colonic electrolyte transport revisited. Gastroenterology 111(5):1358–1368. https://doi.org/10.1053/gast.1996.v111.agast961111358
Charney AN, Egnor RW, Alexander-Chacko J, Cassai N, Sidhu GS (2002) Acid–base effects on intestinal Na+ absorption and vesicular trafficking. Am J Physiol Cell Physiol 283(3):C971–C979. https://doi.org/10.1152/ajpcell.00079.2002
Charney AN, Egnor RW, Henner D, Rashid H, Cassai N, Sidhu GS (2004) Acid–base effects on intestinal Cl– absorption and vesicular trafficking. Am J Physiol Cell Physiol 286(5):C1062–C1070. https://doi.org/10.1152/ajpcell.00454.2003
Freel RW, Whittamore JM, Hatch M (2013) Transcellular oxalate and Cl– absorption in mouse intestine is mediated by the DRA anion exchanger Slc26a3, and DRA deletion decreases urinary oxalate. Am J Physiol Gastrointest Liver Physiol 305(7):G520–G527. https://doi.org/10.1152/ajpgi.00167.2013
Freel RW, Hatch M, Green M, Soleimani M (2006) Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol 290(4):G719–G728. https://doi.org/10.1152/ajpgi.00481.2005
Jiang ZR, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS (2006) Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38(4):474–478. https://doi.org/10.1038/ng1762
Whittamore JM, Frost SC, Hatch M (2015) Effects of acid–base variables and the role of carbonic anhydrase on oxalate secretion by the mouse intestine in vitro. Physiol Rep 3(2):e12282. https://doi.org/10.14814/phy2.12282
Hafner P, Grimaldi R, Capuano P, Capasso G, Wagner CA (2008) Pendrin in the mouse kidney is primarily regulated by Cl– excretion but also by systemic metabolic acidosis. Am J Physiol Cell Physiol 295(6):C1658–C1667. https://doi.org/10.1152/ajpcell.00419.2008
Alesutan I, Daryadel A, Mohebbi N, Pelzl L, Leibrock C, Voelkl J, Bourgeois S, Dossena S, Nofziger C, Paulmichl M, Wagner CA, Lang F (2011) Impact of bicarbonate, ammonium chloride, and acetazolamide on hepatic and renal SLC26A4 expression. Cell Physiol Biochem 28(3):553–558. https://doi.org/10.1159/000335114
Green ML, Hatch M, Freel RW (2005) Ethylene glycol induces hyperoxaluria without metabolic acidosis in rats. Am J Physiol Renal Physiol 289(3):F536–F543. https://doi.org/10.1152/ajprenal.00025.2005
Whittamore JM, Hatch M (2017) Loss of the anion exchanger DRA (Slc26a3), or PAT1 (Slc26a6), alters sulfate transport by the distal ileum and overall sulfate homeostasis. Am J Physiol Gastrointest Liver Physiol 313(3):G166–G179. https://doi.org/10.1152/ajpgi.00079.2017
Nowik M, Kampik NB, Mihailova M, Eladari D, Wagner CA (2010) Induction of metabolic acidosis with ammonium chloride (NH4Cl) in mice and rats—species differences and technical considerations. Cell Physiol Biochem 26(6):1059–1072. https://doi.org/10.1159/000323984
Ribayamercado JD, Gershoff SN (1984) Effects of sugars and vitamin-b-6 deficiency on oxalate synthesis in rats. J Nutr 114(8):1447–1453
Bushinsky DA, Grynpas MD, Asplin JR (2001) Effect of acidosis on urine supersaturation and stone formation in genetic hypercalciuric stone-forming rats. Kidney Int 59(4):1415–1423. https://doi.org/10.1046/j.1523-1755.2001.0590041415.x
Iversen NK, Malte H, Baatrup E, Wang T (2012) The normal acid base status of mice. Respir Physiol Neurobiol 180(2–3):252–257. https://doi.org/10.1016/j.resp.2011.11.015
Freel RW, Hatch M, Earnest DL, Goldner AM (1980) Oxalate transport across the isolated rat colon. A re-examination. Biochem Biophys Acta 600(3):838–843
Hatch M, Freel RW (2003) Renal and intestinal handling of oxalate following oxalate loading in rats. Am J Nephrol 23(1):18–26. https://doi.org/10.1159/000066300
Singh AK, Sjoblom M, Zheng W, Krabbenhoft A, Riederer B, Rausch B, Manns MP, Soleimani M, Seidler U (2008) CFTR and its key role in in vivo resting and luminal acid-induced duodenal HCO3 – secretion. Acta Physiol 193(4):357–365. https://doi.org/10.1111/j.1748-1716.2008.01854.x
Wagner JD, Kurtin P, Charney AN (1985) Effect of systemic acid–base-disorders on colonic intracellular ph and ion-transport. Am J Physiol Gastrointest Liver Physiol 249(1):G39–G47
Feldman GM (1989) Effect of chronic metabolic-acidosis on net electrolyte transport in rat colon. Am J Physiol Gastrointest Liver Physiol 256(6):G1036–G1040
Goldfarb DS, Sly WS, Waheed A, Charney AN (2000) Acid–base effects on electrolyte transport in CA II-deficient mouse colon. Am J Physiol Gastrointest Liver Physiol 278(3):G409–G415
Charney AN, Egnor RW, Steinbrecher KA, Cohen MB (2004) Effect of secretagogues and pH on intestinal transport in guanylin-deficient mice. Biochim Biophys Acta Gen Subj 1671(1–3):79–86. https://doi.org/10.1016/j.bbagen.2004.01.007
Brion LP, Cammer W, Satlin LM, Suarez C, Zavilowitz BJ, Schuster VL (1997) Expression of carbonic anhydrase IV in carbonic anhydrase II-deficient mice. Am J Physiol Renal Physiol 273(2):F234–F245
Lien YHH, Lai LW (1998) Respiratory acidosis in carbonic anhydrase II-deficient mice. Am J Physiol Lung Cell Mol Physiol 274(2):L301–L304
Packer RK, Curry CA, Brown KM (1995) Urinary organic anion excretion in response to dietary acid and base loading. J Am Soc Nephrol 5(8):1624–1629
Knight TF, Senekjian HO, Weinman EJ (1979) Effect of para-aminohippurate on renal transport of oxalate. Kidney Int 15(1):38–42. https://doi.org/10.1038/ki.1979.5
Lemann J, Gray RW, Pleuss JA (1989) Potassium bicarbonate, but not sodium-bicarbonate, reduces urinary calcium excretion and improves calcium balance in healthy-men. Kidney Int 35(2):688–695. https://doi.org/10.1038/ki.1989.40
Lemann J, Hornick LJ, Pleuss JA, Gray RW (1989) Oxalate is overestimated in alkaline urines collected during administration of bicarbonate with no specimen pH adjustment. Clin Chem 35(10):2107–2110
Mazzachi BC, Teubner JK, Ryall RL (1984) Factors affecting measurement of urinary oxalate. Clin Chem 30(8):1339–1343
Chalmers AH, Cowley DM, McWhinney BC (1985) Stability of ascorbate in urine—relevance to analyses for ascorbate and oxalate. Clin Chem 31(10):1703–1705
Fuller NJ, Elia M (1988) Factors influencing the production of creatinine - implications for the determination and interpretation of urinary creatinine and creatine in man. Clin Chim Acta 175(3):199–210. https://doi.org/10.1016/0009-8981(88)90096-4
Miki K, Sudo A (1998) Effect of urine pH, storage time, and temperature on stability of catecholamines, cortisol, and creatinine. Clin Chem 44(8):1759–1762
Wyss M, Kaddurah-Daouk R (2000) Creatine and creatinine metabolism. Physiol Rev 80(3):1107–1213
Perrone RD, Madias NE, Levey AS (1992) Serum creatinine as an index of renal-function - new insights into old concepts. Clin Chem 38(10):1933–1953
Hamm LL, Simon EE (1987) Roles and mechanisms of urinary buffer excretion. Am J Physiol Renal Physiol 253(4):F595–F605
Wamberg S, Hansen AC, Engel K, Kildeberg P (1978) Balance of net base in rat. 3. Effects of oral sodium-bicarbonate and sodium citrate loading. Biol Neonate 34(1–2):24–31
Oster JR, Stemmer CL, Perez GO, Vaamonde CA (1988) Comparison of the effects of sodium-bicarbonate versus sodium-citrate on renal acid excretion. Miner Electrol Metab 14(2–3):97–102
Brown JC, Packer RK, Knepper MA (1989) Role of organic-anions in renal response to dietary acid and base loads. Am J Physiol Renal Physiol 257(2):F170–F176
Eladari D, Leviel F, Pezy F, Paillard M, Chambrey R (2002) Rat proximal NHE3 adapts to chronic acid–base disorders but not to chronic changes in dietary NaCl intake. Am J Physiol Renal Physiol 282(5):F835–F843. https://doi.org/10.1152/ajprenal.00188.2001
Lemann J, Lennon EJ, Goodman AD, Litzow JR, Relman AS (1965) Net balance of acid in subjects given large loads of acid or alkali. J Clin Investig 44(4):507–517. https://doi.org/10.1172/jci105164
Hood VL (1985) pH regulation of endogenous acid production in subjects with chronic ketoacidosis. Am J Physiol Renal Physiol 249(2):F220–F226
Lindinger MI, Franklin TW, Lands LC, Pedersen PK, Welsh DG, Heigenhauser GJF (2000) NaHCO3 and KHCO3 ingestion rapidly increases renal electrolyte excretion in humans. J Appl Physiol 88(2):540–550
Khanna A, Kurtzman NA (2006) Metabolic alkalosis. J Nephrol 19:S86–S96
Maren TH (1967) Carbonic anhydrase—chemistry physiology and inhibition. Physiol Rev 47(4):595–781
Swenson ER, Maren TH (1978) Quantitative-analysis of CO2 transport at rest and during maximal exercise. Respir Physiol 35(2):129–159. https://doi.org/10.1016/0034-5687(78)90018-x
Harrison HE, Harrison HC (1955) Inhibition of urine citrate excretion and the production of renal calcinosis in the rat by acetazolamide (Diamox) administration. J Clin Investig 34(11):1662–1670. https://doi.org/10.1172/jci103220
Hamm LL (1990) Renal handling of citrate. Kidney Int 38(4):728–735. https://doi.org/10.1038/ki.1990.265
Welch BJ, Graybeal D, Moe OW, Maalouf NM, Sakhaee K (2006) Biochemical and stone-risk profiles with topiramate treatment. Am J Kidney Dis 48(4):555–563. https://doi.org/10.1053/j.ajkd.2006.07.003
Ohana E, Shcheynikov N, Moe OW, Muallem S (2013) SLC26A6 and NaDC-1 transporters interact to regulate oxalate and citrate homeostasis. J Am Soc Nephrol 24(10):1617–1626
Maren TH (1977) Use of inhibitors in physiological studies of carbonic-anhydrase. Am J Physiol Renal Physiol 232(4):F291–F297
Matlaga BR, Shah OD, Assimos DG (2003) Drug-induced urinary calculi. Rev Urol 5(4):227–231
Daudon M, Jungers P (2004) Drug-induced renal calculi—epidemiology, prevention and management. Drugs 64(3):245–275. https://doi.org/10.2165/00003495-200464030-00003
Boer P, Beutler JJ, Vanrijn HJM, Berckmans RJ, Koomans HA, Mees EJD (1990) Urinary oxalate excretion during intravenous-infusion of diuretics in man. Nephron 54(2):187–188. https://doi.org/10.1159/000185846
Higashihara E, Nutahara K, Takeuchi T, Shoji N, Araie M, Aso Y (1991) Calcium-metabolism in acidotic patients induced by carbonic-anhydrase inhibitors—responses to citrate. J Urol 145(5):942–948
Parikh JR, Nolan RL (1994) Acetazolamide-induced nephrocalcinosis. Abdom Imaging 19(5):466–467. https://doi.org/10.1007/bf00206942
Mirza N, Marson AG, Pirmohamed M (2009) Effect of topiramate on acid–base balance: extent, mechanism and effects. Br J Clin Pharmacol 68(5):655–661. https://doi.org/10.1111/j.1365-2125.2009.03521.x
Kaplon DM, Penniston KL, Nakada SY (2011) Patients with and without prior urolithiasis have hypocitraturia and incident kidney stones while on topiramate. Urology 77(2):295–298. https://doi.org/10.1016/j.urology.2010.06.048
Acknowledgements
The authors wish to thank Maureen Mohan for excellent technical assistance and animal husbandry.
Funding
This work was supported by NIH grants DK108755 and DK088892 to M. Hatch, and an Experimental Pathology Innovative Grant from the Department of Pathology, Immunology and Laboratory Medicine in the UF College of Medicine to J. Whittamore.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experimental procedures were undertaken in accordance with the ethical standards of the University of Florida Institutional Animal Care and Use Committee (IACUC) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Rights and permissions
About this article
Cite this article
Whittamore, J.M., Hatch, M. Oxalate transport by the mouse intestine in vitro is not affected by chronic challenges to systemic acid–base homeostasis. Urolithiasis 47, 243–254 (2019). https://doi.org/10.1007/s00240-018-1067-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-018-1067-5