Abstract
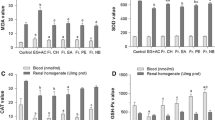
Desmosium styracifolium (D. styracifolium), which is considered as a Chinese herbal medicine, has been reported to treat the kidney stone diseases. However, the potential phytochemically active components and the underlying mechanisms associated with its efficacy in targeting urolithiasis remain to be elucidated. This study aims to investigate the anti-urolithiatic effect of total flavonoids of D. styracifolium (TFDS) on calcium oxalate (CaOx) renal stones in Sprague–Dawley rats. Animal models of CaOx urolithiasis were established in male Sprague–Dawley rats by adding 5% w/w hydroxy-l-proline (HLP) in regular rat chow. The TFDS orally at 100, 400 mg/kg, respectively, were administered along with HLP for 28 days. At the end of 28 days of treatment, urine and serum samples were collected for crystalluria determination and various biochemical analysis. Kidney tissues were isolated and processed for antioxidant parameters measurement and histopathological examinations. HLP-induced hyperoxaluria alone reliably caused CaOx nephrolithiasis in rats. We showed that TFDS significantly reduced crystalluria and CaOx crystal deposits in the kidney sections as compared to untreated HLP group. Also, TFDS was observed to decrease urinary oxalate excretion, alleviate the pro-acidosis condition, improve the impaired renal functions and renal epithelial cell injury. Moreover, TFDS protected against the oxidative stress changes via reducing MDA content, increasing CAT and GSH-Px activities in renal homogenate, as well as attenuating the expression of MCP-1, OPN and TGF-β proteins. These results indicated that TFDS had beneficial effect on inhibition of CaOx formation in the rat kidney probably through a combination of antioxidant, anti-inflammatory, urine alkalinizing activities, and lowering the concentration of urinary stone-forming constituents. Thus, TFDS might have clinical implications in preventing oxidative renal cell injury and, ultimately, kidney stone formation. The data provide a rationale for the medicinal use of TFDS in nephrolithiasis and identify this agent as a potential source of new antiurolithic drugs.





Similar content being viewed by others
References
Edvardsson VO, Indridason OS, Haraldsson G, Kjartansson O, Palsson R (2013) Temporal trends in the incidence of kidney stone disease. Kidney Int 83(1):146–152
Vanachayangkul P, Byer K, Khan S, Butterweck V (2010) An aqueous extract of Ammi visnaga fruits and its constituents khellin and visnagin prevent cell damage caused by oxalate in renal epithelial cells. Phytomedicine 17(8–9):653–658
Moe OW (2006) Kidney stones: pathophysiology and medical management. Lancet 367(9507):333–344
Gurocak S, Kupeli B (2006) Consumption of historical and current phytotherapeutic agents for urolithiasis: a critical review. J Urol 176(2):450–455
Butterweck V, Khan SR (2009) Herbal medicines in the management of urolithiasis: alternative or complementary? Planta Med 75(10):1095–1103
Miyaoka R, Monga M (2009) Use of traditional Chinese medicine in the management of urinary stone disease. Int Braz J Urol 35(4):396–405
Zhao M, Duan JA, Che CT (2007) Isoflavanones and their O-glycosides from Desmodium styracifolium. Phytochemistry 68(10):1471–1479
Zhang YQ, Luo JG, Han C, Xu JF, Kong LY (2015) Bioassay-guided preparative separation of angiotensin-converting enzyme inhibitory C-flavone glycosides from Desmodium styracifolium by recycling complexation high-speed counter-current chromatography. J Pharm Biomed Anal 102:276–281
Gohel MD, Wong SP (2006) Chinese herbal medicines and their efficacy in treating renal stones. Urol Res 34(6):365–372
Mi J, Duan J, Zhang J, Lu J, Wang H, Wang Z (2012) Evaluation of antiurolithic effect and the possible mechanisms of Desmodium styracifolium and Pyrrosiae petiolosa in rats. Urol Res 40(2):151–161
Xiang S, Zhou J, Gan S, Rong XL, Li J, Wang S (2013) Antiurolithiatic activity of aqueous extract from Desmodium styracifolium (Osb.) Merr. on renal calcium oxalate in rats. Chin J Exp Surg 30(6):1166
Xiang S, Gan S, Zhou J et al (2014) Effects of aqueous extract from Desmodium styracifolium (Osb.) Merr. on oxidative stress of renal calcium oxalate rat models. Chin J Urol 35(6):465–468
Xiang S, Zhou J, Li J et al (2015) Antilithic effects of extracts from different polarity fractions of Desmodium styracifolium on experimentally induced urolithiasis in rats. Urolithiasis 43(5):433–439
Khan SR (2013) Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol 189(3):803–811
Khan SR (2005) Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res 33(5):349–357
Tsujihata M (2008) Mechanism of calcium oxalate renal stone formation and renal tubular cell injury. Int J Urol 15(2):115–120
Umekawa T, Iguchi M, Uemura H, Khan SR (2006) Oxalate ions and calcium oxalate crystal-induced up-regulation of osteopontin and monocyte chemoattractant protein-1 in renal fibroblasts. BJU Int 98(3):656–660
Tsujihata M, Yoshioka I, Tsujimura A, Nonomura N, Okuyama A (2011) Why does atorvastatin inhibit renal crystal retention? Urol Res 39(5):379–383
Zhang L, Han J, Feng Z, Li Q, Guan S, Liao Q (2011) Enrichment and purification technology for total flavonoids from aerial part of Desmodium styracifolium by macroporous resin. Chin Tradit Herbal Drugs 42(12):2442–2446
Khan SR, Glenton PA, Byer KJ (2006) Modeling of hyperoxaluric calcium oxalate nephrolithiasis: experimental induction of hyperoxaluria by hydroxy-l-proline. Kidney Int 70(5):914–923
Joshi S, Saylor BT, Wang W, Peck AB, Khan SR (2012) Apocynin-treatment reverses hyperoxaluria induced changes in NADPH oxidase system expression in rat kidneys: a transcriptional study. PLoS ONE 7(10):e47738
Li CY, Deng YL, Sun BH (2009) Effects of apocynin and losartan treatment on renal oxidative stress in a rat model of calcium oxalate nephrolithiasis. Int Urol Nephrol 41(4):823–833
Bashir S, Gilani AH, Siddiqui AA et al (2010) Berberis vulgaris root bark extract prevents hyperoxaluria induced urolithiasis in rats. Phytother Res 24(8):1250–1255
Zhou C, Luo JG, Kong LY (2012) Quality evaluation of Desmodium styracifolium using high-performance liquid chromatography with photodiode array detection and electrospray ionisation tandem mass spectrometry. Phytochem Anal 23(3):240–247
Yoshioka I, Tsujihata M, Akanae W, Nonomura N, Okuyama A (2011) Angiotensin type-1 receptor blocker candesartan inhibits calcium oxalate crystal deposition in ethylene glycol-treated rat kidneys. Urology 77(4): 1007e9-1007e14
Park S, Pearle MS (2007) Pathophysiology and management of calcium stones. Urol Clin North Am 34(3):323–334
Cho HJ, Bae WJ, Kim SJ et al (2014) The inhibitory effect of an ethanol extract of the spores of Lygodium japonicum on ethylene glycol-induced kidney calculi in rats. Urolithiasis 42(4):309–315
Khan A, Khan SR, Gilani AH (2012) Studies on the in vitro and in vivo antiurolithic activity of Holarrhena antidysenterica. Urol Res 40(6):671–681
Bayir Y, Halici Z, Keles MS et al (2011) Helichrysum plicatum DC. subsp. plicatum extract as a preventive agent in experimentally induced urolithiasis model. J Ethnopharmacol 138(2):408–414
Park HK, Jeong BC, Sung MK et al (2008) Reduction of oxidative stress in cultured renal tubular cells and preventive effects on renal stone formation by the bioflavonoid quercetin. J Urol 179(4):1620–1626
Acknowledgements
We are grateful to Dr. Wenqi Wu (Guangdong Key Laboratory of Urology, Minimally Invasive Surgery Center, the First Affiliated Hospital of Guangzhou Medical University, China) for the kind assistance in this study. This work was supported in part by the Science and Technology Planning Project of Guangdong Province (Grant Nos. 2011B061300077, 2008B030301363), and the Key Project of Traditional Chinese Medicine Bureau of Guangdong Province (Grant No. 20173009), “The Construction of High-level University” Public Projects from Guangzhou University of Chinese Medicine (Grant No. 2017B022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential financial competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, J., Jin, J., Li, X. et al. Total flavonoids of Desmodium styracifolium attenuates the formation of hydroxy-l-proline-induced calcium oxalate urolithiasis in rats. Urolithiasis 46, 231–241 (2018). https://doi.org/10.1007/s00240-017-0985-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-017-0985-y