Abstract
Purpose
A combination of the caloric test with functional magnetic resonance imaging (fMRI) is a promising method for a comprehensive diagnostics of pathologies of the vestibular system. The aim of this study was to investigate the potential pattern of grey matter local activation in fMRI using cold and hot caloric stimulation in patients presenting unilateral peripheral vestibular injury.
Methods
Forty right-handed participants aged 27 to 56 with the diagnosis of right-sided peripheral vestibular hypofunction were included. Stimulation was performed separately for the right and the left ear with cold (C, 14–15 °C) stimulus and hot (H, 48–49 °C) stimulus. Grey matter activation was assessed in BOLD technique using a 3T scanner.
Results
We observed activity within the parieto-insular vestibular cortex (PIVC), thalamus, insula and retroinsular area, hippocampus, and cerebellum, as well as oculomotor centers located in the precentral gyrus, superior temporal gyrus, and intraparietal sulcus. Cold stimulus resulted in more areas of activation in response to the right ear activation rather than to the left ear. The ipsilateral activity was noted for insular cortex and intraparietal sulcus. The differences between hot and cold stimuli were noted for the right ear.
Conclusions
In this preliminary study, the combination of the caloric test and fMRI allowed to present specific pattern of grey matter activation in patients with unilateral peripheral vestibular injury. Further studies are necessary to develop patterns or cortical maps differentiating various balance disorders and to analyze the dynamics of cortical plasticity after the injury.
Similar content being viewed by others
Introduction
The sense of balance is a major source of information about body position in space and its acceleration. The feeling of movement is derived from the information input from topographically and functionally distant brain cortical areas, particularly the vestibular, vision, and somatosensory systems. Each element contains neural pathways and a specific cortical area, often scattered in a couple of locations. This complexity leads to difficulties in diagnosis and treatment of vertigo and balance disorders. Moreover, “dizziness” is a commonly used umbrella term encompassing vertigo, disequilibrium, and pre-syncope as well as other non-specific symptoms [1,2,3,4,5].
The least common method for vestibular stimulation in functional magnetic resonance imaging (fMRI) is high-intensity sound stimulation. The sound excites the inner ear otolith organ and triggers postural muscle reflexes, including the sternocleidomastoid and oculomotor muscles, which form so-called vestibular evoked myogenic potentials (VEMP) [6,7,8]. Recent studies on animals show that the acoustic stimuli can activate both otolith organ neurons and semicircular canals [9, 10]. The acoustic fMRI test requires scrupulous separation of acquired data from scanner calibration due to similarities between both sounds.
The second and more commonly used method of vestibular stimulation in fMRI is the galvanic test, during which electric current is conducted between the mastoid processes. This type of stimulation causes hyperpolarization of the vestibular epithelium with no effect on hairy cells [6] although reports on this result are contradictory [11]. Activation power is greater on the cathode side compared to the anode side; however, all otoliths and afferent neurons from all canals are excited at the same time [6]. The test is accompanied by sensory, thermal, and pain sensation, as well as dizziness, metallic taste, and sometimes nausea. Some researchers additionally applied nuchal skin electric stimulation during fMRI, which was supposed to reduce those sensations [12, 13].
In current otoneurology, the Fitzgerald-Hallpike caloric test with induced nystagmus registration is the most commonly used diagnostic tool to estimate the labyrinth function. In this test, heat transfer from external auditory meatus to the inner ear results in endolymph movement. This results in the change of bioelectric potential of the stimulated semicircular canal, then neuron depolarization, and, finally, increased conduction along the cranial nerve VIII. Lowering temperature causes the endolymph to move in the opposite direction with respect to hot stimulation. Then, nerve VIII conduction is reduced compared to the zero state. The induced stimulus activates the vestibulo-cerebello-oculomotor (vestibulo-oculomotor) and vestibulo-cerebello-spinal reflexes. The first attempts of caloric stimulation in fMRI were limited to one type of stimulus [14, 15]. Recently, Frank and Greenlee proposed a device that offered both cold and hot stimulation but they validated the system only on 13 healthy participants [16]. Therefore, the aim of this preliminary study was to investigate the potential pattern of grey matter local activation in fMRI using cold and hot caloric stimulation in patients presenting unilateral peripheral vestibular injury.
Materials and methods
Caloric stimulation device
For the study, we used a device for caloric vestibular stimulation in fMRI designed by Frank and Greenlee [16] and modified by our team. We used the inner ear temperature change as the stimulus, administering either very hot (H) or cold (C) water to the ear external canal. The neutral stimulus was temperature close to physiological body temperature, which was achieved by infusing warm water (W). The device utilized closed circulation of water at a given temperature. The water was delivered from outside of the Faraday cage from the control room with pumps to the glass capsules placed inside the ears of the patient, who was lying down on the MR scanner’s table. The device scheme of the device is shown in Fig. 1.
Additionally, during the caloric test, the induced nystagmus of the right eye was registered. The registration was made at a frequency of 50 Hz using an infrared camera (Visual System, NordicNeuroLab, Bergen, Norway). In both oculars, black background was used (no eye fixation). The analysis of registered data was conducted using the ViewPoint EyeTracker® software (Arrington Research, Inc., Scottsdale, USA).
Device calibration
Before commencing the experiments, it was necessary to calibrate the initial temperature of hot and cold water and the temperature in the glass capsules. In order to do this, we performed a series of 40 temperature measurements outside the Faraday cage at the same physical conditions.
To trigger the vestibulo-cerebello-oculomotor (vestibulo-oculomotor) and vestibulo-cerebello-spinal reflexes, it was necessary to apply temperature higher or lower relative to the body temperature; in the Fitzgerald-Hallpike test, the difference should be at least of 7 °C, i.e., not higher than 30 °C for cold water and not lower than 44 °C for hot water. The water temperature was controlled before each study using a digital K-type multimeter (VOLCRAFT MT-52, China). Based on a series of calibration measurements, we determined that the water temperature in the container must be 13 °C and 50 °C, respectively, in order to conduct the experiment and achieve water temperatures in the capsules of 14–15 °C and 48–49 °C accordingly. In order to habituate the patient to the noise of flowing water, we used a 60-s neutral stimulus with body temperature (37 °C) before caloric stimulation.
The effect of temperature on MRI signal in BOLD sequence and artifact creation was described by Lobel et al. and Rieke et al. [12, 17]. In their studies, they pointed out that some imaging parameters are sensitive to surrounding temperature. To better understand this problem and to eliminate it during patient tests, we conducted two additional experiments using an agar dummy (1% agar with 2 mM NiCl2). In the first test, we evaluated the dummy image in response to the neutral stimulus (W). In the second test, we conducted a full assessment (structural and functional sequence) with three stimuli: stimulus H to the left dummy pole, stimulus C to the right dummy pole, and stimulus W to both poles. It was determined that cold water had a greater effect on BOLD signal compared to hot and warm water, which both triggered similar effects. The signals were located superficially near the capsules. Therefore, it can be assumed that in vivo caloric artifacts do not map onto the brain and do not distort results.
Study group
The size of the study group was calculated based on a study by Mumord [18]. We included 40 right-handed participants aged 27 to 56 (mean age 39) with the diagnosis of right-sided peripheral vestibular hypofunction (Table 1). The diagnosis was made by the laryngologist during standard otoneurological diagnostic procedures (videonystagmography, posturography, vestibular evoked myogenic potentials, temporal bone CT, and brain MRI). The diagnostic procedure was the same for all the participants. Videonystagmography was performed using a VNG Ulmer system (Synapsys, France). The following vestibulo-oculomotor and vestibulo-visual reactions were assessed: saccadic eye movements, optokinetic reaction, smooth pursuit movements, positional nystagmus, gaze nystagmus, spontaneous nystagmus, cervical nystagmus, and caloric bithermal reaction. For posturography, a SPS system (Synapsys, France) was used to test sensory organization with the estimation of somatosensory, visual, and vestibular efficiency, which simultaneously constitute global postural effect (final stabilogram). Vestibular evoked myogenic potentials were stimulated using Centor C+ system (Deltamed, France) by presenting a 110-dB and 500-Hz sound wave to each ear and collected ipsilaterally and contralaterally from the sternocleidomastoid muscle. The latency of the P13N23P30 waves, their morphology, and amplitudes were analyzed.
Exclusion criteria included the following: psychiatric or neurological disorders, temporal bone pathologies seen on CT, brain tumors or malacia on MRI, previous brain or ear surgery, pregnancy, claustrophobia, metal foreign objects, or metal implants (heart stimulator, cardioverter, insulin pump, cochlear implant, CNS stimulation, prosthesis). All participants were adults and gave informed consent to participate. We obtained a permission to conduct the study from the local bioethical committee.
Scanning
The study was conducted at the Interdisciplinary Center for Modern Technology, Nicolaus Copernicus University, Toruń, Poland, using a 3T MRI scanner (GE Discovery MR 750, General Electric Healthcare, USA) and 8-channel neurovascular head coil (HD 8-CH Neurovascular Array, General Electric Healthcare, USA). After installing the capsules and the eye-tracker, the patient was placed in the MRI gantry in a supine position with his/her head tilted at 30° towards the chest. The reproducibility of the head position and the alignment of the canal with gravitational force vector were achieved using a pillow wedge. During the experiment, the scanner room was dark to minimize eye fixation.
For each participant, experimentation started with a volumetric high-definition brain scanning in 3D BRAVO sequence (3D T1W1, TR–10.4, TE–4.4, FA–90°, thickness–1.2 mm, gap–0.0). After structural imaging, the functional study was performed using BOLD technique in axial plane using EPI sequence (TR–2000, TE–27, FA–90°, layer thickness–3.0 mm, gap–0.0). The EPI sequence lasted 13 min on average. Two sessions were conducted; during the first session, hot water stimulation was used (four stimulations to the right ear and four stimulations to the left ear), while cold water was used during the second session. Thus, each session contained nine alternating blocs of 30-s stimulation and 60-s rest (time of registration was calculated on the base of oculomotor reaction during F-H test, with peak expected between 60 and 90 s after beginning of stimulation). This process allowed for data acquisition at the maximum BOLD reaction time. The effect of habituation, usually present during stimulus repetition, was not essential and may result only in smaller reaction, not its absence.
Data analysis
Data preprocessing and analysis were conducted using a toolkit FSL v. 5.0 (The FMRIB Software Library). The image quality control allowed for evaluation of fMRI scans for field distortion and spike artifacts, which were not found, and also to verify the mean voxel intensity to intensity variance in the next volume ratio (temporal signal-to-noise ratio, tSNR). All EPI scans were also subject to the correction of movement, acquisition time, coregistration, normalization, segmentation, and spatial smoothing. Structural imaging transform coefficient was used for functional studies. The main method for functional image analysis was the subtraction of mean signal value during activation from the mean control signal value. This process was applied to each voxel, which created the activity map. In practice, however, this method is avoided in group analysis due to a considerable systematic bias, and more sophisticated algorithms are preferred. For structural and functional image registration to the spatial atlas MNI152, we used the FLIRT library (FMRIB’s Linear Image Registration Tool) [19].
The fMRI data analysis was conducted using FEAT library v. 6.00 (FMRI Expert Analysis Tool). Movement correction was conducted using MCFLIRT package [20], while spatial smoothing was conducted using Gaussian filter (FWHM 4.0 Mm). We also used high-pass filter in time domain (Gaussian-weighted least-squares straight line fitting, sigma = 50.0S). Time series analysis was conducted using FILM software (FMRIB’s Improved Linear Model) with local correlation correction [21, 22].
Statistical analysis
Z statistic images (Gaussianized T/F) were set in terms of clusters for Z > 2.8. The cluster significance (with correction) was set at p = 0.05. The first five EPI scans were discarded in each data package to achieve signal balance. For fMRI image coregistration, we used structural 3D T1W1 scans in BRAVO sequence. The first-level statistical analysis (individual data) was conducted using a general linear model (GLM). The second-level statistical analysis (group analysis) for difference assessment between C and H stimuli was conducted using FLAME library (FMRIB’s Local Analysis of Mixed Effects), which is a type of variance test for mixed effects in a full Bayesian network.
Results
None of the participants stopped the procedure due to discomfort during stimulation or complained about significant side effects of caloric stimulation (e.g., annoying nausea, vomiting, palpitation, vertigo). In order to confirm the effectiveness of caloric stimulation during the experiment, the right eye nystagmus was registered in addition to subjective vertigo sensation. We only registered the presence of nystagmus and its detailed characteristics were not subject to analysis.
The device effectiveness was confirmed in all patients: hot water stimulation led to nystagmus towards the stimulated ear while cold water stimulation occurred in the opposite direction. Cold stimulus resulted in more areas of activation in response to right ear activation rather than to the left ear. The differences between hot and cold stimuli were noted for the right ear.
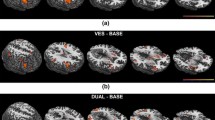
We observed activity within the parieto-insular vestibular cortex (PIVC), thalamus, insula and retroinsular areas, hippocampus, and cerebellum as well as oculomotor centers located in the precentral gyrus, superior temporal gyrus, and intraparietal sulcus. Significant activity in response to neutral stimulus to both ears was noted in the right fronto-parieto-temporal area, hippocampus, and cerebellar hemisphere, which may be considered a measurement of noise. Figures 2, 3, 4, and 5 depict activation of the above-listed structures. Tables 2, 3, 4, and 5 show brain areas in patients that presented statistically significant activity in response to left and right ear caloric stimulation.
Graphical presentation of brain areas statistically activated after applying cold stimulus to the right ear. Significant activations (red areas) are listed in Table 2. The remaining indicated areas were not statistically significant
Graphical presentation of brain areas statistically activated after applying cold stimulus to the left ear. Significant activations (red areas) are listed in Table 3. The remaining indicated areas were not statistically significant
Graphical presentation of brain areas statistically activated after applying hot stimulus to the right ear. Significant activations (red areas) are listed in Table 4. The remaining indicated areas were not statistically significant
Graphical presentation of brain areas statistically activated after applying hot stimulus to the left ear. Significant activations (red areas) are listed in Table 5. The remaining indicated areas were not statistically significant
Discussion
In this preliminary study, we presented the potential pattern of grey matter local activation in fMRI using cold and hot caloric stimulation in patients presenting unilateral vestibular injury. We observed increased activity within the parieto-insular vestibular cortex (PIVC), thalamus, insula and retroinsular areas, hippocampus, and cerebellum as well as oculomotor centers in the precentral gyrus, superior temporal gyrus, and intraparietal sulcus. We noticed significantly increased activity in response to the neutral stimuli to both ears within the right fronto-parieto-temporal cortex, hippocampus, and cerebellar hemispheres. The activity may be considered a noise from the method or measurement itself and may be attributed to scanning-related stress, scanner’s noise, sensation of water flowing, and touch sensation from the external ears. In most tests, increased activity was observed within the insula, especially the parietal operculum, which is in accordance with previous studies. Many more centers were stimulated by the cold stimulus applied to the right ear rather than left ear and ipsilateral activity was observed within the insula and intraparietal sulcus. The differences between the hot and cold stimuli were more pronounced for the right ear.
Numerous human and animal studies demonstrated the activation of several separate and distinct cortical areas during the processing of vestibular information [13,14,15,16]. Evidence from functional imaging studies has revealed a complex network of cortical areas in both human hemispheres, predominantly in the temporo-insular and temporo-parietal cortex [14]. It has been proven that vestibular stimulation modulates somatosensory cortical processing via the activation of the parietal operculum [17]. The region of the frontal lobe, which participates in the vestibular processing, is an origin of the fronto-ponto-cerebellar tract [18]. The damage of this pathway may result in cerebellar ataxia. Patients with neurological deficits have been noted to be hyporesponsive to the proprioceptive and vestibular stimuli. Cognitive dysfunction due to the damage of frontal, temporal, and parietal cortex may disturb the stimuli-responsivity, vestibular compensation, and reactivity [19]. In previous attempts to map the vestibular cortex, only pathways engaged in stimulus processing were mentioned. Depending on the vestibular stimulation and registration methods, various patterns of spontaneous activity in term of location and extent were observed. Moreover, the activity was not symmetrical in both hemispheres. Higher activity was observed in the non-dominant hemisphere and on the same side as the stimulus [6, 12, 13, 23]. The first attempts of functional imaging with caloric stimulation [14,15,16] were limited mainly to healthy individuals and one type of stimulus. In the study by Suzuki et al., vestibular stimulation of the right ear with cold water activated the left superior temporal gyrus as well as the right superior temporal gyrus, right intraparietal sulcus, right parahippocampal gyrus, right hippocampus, and left insula; the highest Z value denoting the highest activity agreement between subjects was mapped in the left superior temporal gyrus [15].
In response to left ear stimulation, we observed an increased activity of the right cingulate gyrus, right superior temporal gyrus, left hippocampus and parahippocampal gyri, left intraparietal sulcus, right cingulate gyrus, and right insula. The highest Z value was mapped in the right intraparietal sulcus and right thalamus. In contrast, in the previously mentioned study by Fasold et al. [24], the cold stimulus applied to the right ear caused activation of the left dorsolateral parietal cortex, right dorsolateral parietal cortex, left and right parieto-insular cortices, left superior temporal gyrus, right and left cingulate gyri, left posterior cingulate gyrus, right parieto-frontal operculum, and left prefrontal cortex.
In our study during the left vestibular stimulation, the BOLD signal increase was noted within the right and left posterior insulas, right and left posterior cingulate gyri, left prefrontal cortex, and right superior temporal gyrus. In general, in patients with right-sided vestibular hypofunction, the stimulation of the injured labyrinth typical vestibular still resulted in activation of areas that were identified in previous studies as the vestibular cortex in healthy subjects. However, in our patients, the response for stimulation of the contralateral (healthy) vestibulum included more areas, which were spread outside PIVC. This observation may be interpreted as a plasticity of the whole vestibular system. Since the left ear has to take over the balance control of the body, it also activates new cortical areas. However, these results should be interpreted with caution due to small population size and simplified data analysis. In our study, we confirmed reports by other authors regarding cortical representation of the balance sense in healthy individuals. In previous studies using galvanic stimulation, usually both semicircular canals and otolith organs were stimulated simultaneously. Moreover, the response time was shorter than for caloric stimulation [6, 13]. Depending on current intensity, we observed galvanic skin tension and pain as side effects. These reactions involve the sensory system, which could influence the response of the vestibular system to greater extent than during the caloric test [6, 14]. It can be assumed that, for this reason, galvanic stimulation is associated with a risk of “overlapping” activation of vestibular and somatosensory cortices. Another premise for inequality between galvanic and caloric stimulation is the eye reaction. Galvanic stimulation induces rotational eye movement and the horizontal nystagmus has a smaller amplitude than in the caloric test [12]. Finally, the differences can be attributed to anatomical variants of PIVC (temporo-parietal and temporo-insular cortices). Therefore, direct comparison of the literature data can be challenging.
Our study has some limitations. Firstly, we did not assess the temporal relation between the duration of stimulation and the cortical activation seen in BOLD sequence. In a standard Fitzgerald-Hallpike caloric test, there is a 10-s delay between the start of stimulation and the response, i.e., nystagmus. However, the most intense reaction is after 60–90 s. In fMRI, since BOLD signal is relatively weak, several repeated acquisitions are necessary to gain an image. Therefore, we had to weigh between the total scanning time acceptable for subjects and the strength of the BOLD signal. Since it was a preliminary study, we used cold and hot stimuli to both ears. In further experiments, we will be able to limit the range of stimuli in favor of deeper analysis of temporal aspects of the test. Secondly, due to the delay in vestibular activation described above, stimulation in our fMRI paradigm might be too frequent and might result in overlapping cortical activations. Thirdly, our study group may be considered inhomogeneous. We included right-handed subjects with a laryngological diagnosis of right-sided peripheral vestibular hypofunction with no structural abnormalities of the inner ear. Such diagnosis is called a functional vestibular hypofunction, which means that the particular reason of vertigo is not known and may include various reasons. This group of patients, however, is worth deeper diagnostics as the disease is not fully understood. Moreover, cortical activations seem not to depend on the particular reason of peripheral vestibular injury. Finally, our study was a group analysis of cortical activations in response to cortical activation; therefore, results were averaged within the study group and do not bring any diagnosis for a particular patient. Instead, we were looking for differences between the right and the left ear stimulation and between cold and hot stimuli in this cohort. Therefore, our results present a rather basic scientific study in contrast to a complete clinical test.
In conclusion, the combination of the complete caloric test and fMRI is an innovative approach to diagnosis of vertigo and creates new scientific possibilities. Apart from physiological responses to caloric stimulation, which are still not fully understood, fMRI may become a tool to objectively determine the prognosis regarding recovery and could be an indicator of a successful vestibular rehabilitation. Studies on a larger group would allow development of patterns or cortical maps differentiating various balance disorders and to investigate cortical plasticity after injury. Considering technology, further efforts will be focused on automation and computerization of the system to reduce interscan variability to approximate the device to clinical practice.
References
Oguz S, Demirbuken I, Kavlak B, Acar G, Yurdalan SU, Polat MG (2017) The relationship between objective balance, perceived sense of balance, and fear of falling in stroke patients. Top Stroke Rehabil 24:527–532
Stuart DG (2005) Integration of posture and movement: contributions of Sherrington, Hess, and Bernstein. Hum Mov Sci 24:621–643
Neuhauser HK (2016) The epidemiology of dizziness and vertigo. Handb Clin Neurol 137:67–82
Murdin L, Schilder AG (2015) Epidemiology of balance symptoms and disorders in the community: a systematic review. Otol Neurotol 36:387–392
Muncie HL, Sirmans SM, James E (2017) Dizziness: approach to evaluation and management. Am Fam Physician 95:154–162
Black R, Halmagyi G, Thurtell M, Todd MJ, Curthoys IS (2005) The active head-impulse test in unilateral peripheral vestibulopathy. Arch Neurol 62:290–293
Miyamoto T, Fukushima K, Takada T, de Waele C, Vidal PP (2007) Saccular stimulation of the human cortex: a functional magnetic resonance imaging study. Neurosci Lett 423:68–72
Schlindwein P, Bauermann T, Mueller M, Brandt T, Stoeter P, Dieterich M (2008) Cortical representation of saccular vestibular stimulation: VEMPs in fMRI. Neuroimage 39:19–31
Xu Y, Simpson I, Tang X, Zhou W (2009) Acoustic clicks activate both the canal and otolith vestibulo-ocular reflex pathways in behaving monkeys. J Assoc Res Otolaryngol 10:569–577
Zhu H, Tang X, Wei W, Mustain W, Mustain W, Xu Y, Zhou W (2011) Click evoked responses in vestibular afferents in rats. J Neurophysiol 106:754–763
Aw ST, Todd MJ, Aw GE, Weber KP, Halmaqyi GM (2008) Gentamicin vestibulotoxicity impairs human electrically evoked vestibulo-ocular reflex. Neurology 71:1776–1782
Lobel E, Kleine J, Le Bihan D, Leroy-Williq A, Berthoz A (1998) Functional MRI of galvanic vestibular stimulation. J Neurophysiol 80:2699–2709
Bense S, Stephan T, Yousry T, Brandt T, Dieterich M (2001) Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI). J Neurophysiol 85:886–899
Vitte E, Derosier C, Caritu Y, Berthoz A, Hasboun D, Soulié D (1996) Activation of the hippocampal formation by vestibular stimulation: a functional magnetic resonance imaging study. Exp Brain Res 112:523–526
Suzuki M, Kitano H, Ito R, Kitanishi T, Yazawa Y, Ogawa T, Shiino A, Kitajima K (2001) Cortical and subcortical vestibular response to caloric stimulation detected by functional magnetic resonance imaging. Brain Res Cogn Brain Res 12:441–449
Frank SM, Greenlee MW (2014) An MRI-compatible caloric stimulation device for the investigation of human vestibular cortex. J Neurosci Methods 30:208–218
Rieke V, Butts PK (2008) MR thermometry. Magn Reson Imaging 27:376–390
Mumford JA (2012) A power calculation guide for fMRI studies. Soc Cogn Affect Neurosci 7:738–742
Woolrich MW, Ripley BD, Brady JM, Smith SM (2001) Temporal autocorrelation in univariate linear modelling of FMRI data. Neuroimage 14:1370–1386
Jenkinson M, Bannister P, Brady JM, Smith SM (2002) Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841
McIntosh AR, Gonzalez-Lima F (1994) Structural equation modeling and its application to network analysis in functional brain imaging. Hum Brain Mapp 2:2–22
Dale AM (1999) Optimal experimental design for event-related fMRI. Hum Brain Mapp 8:109–114
Fitzpatrick RC, Day BL (2004) Probing the human vestibular system with galvanic stimulation. J Appl Physiol 96:2301–2316
Fasold O, von Brevern M, Kuhberg M, Ploner CJ, Villringer A, Lempert T, Wenzel R (2002) Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging. NeuroImage 17:1384–1393
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wypych, A., Serafin, Z., Marzec, M. et al. Grey matter activation by caloric stimulation in patients with unilateral peripheral vestibular hypofunction. Neuroradiology 61, 585–593 (2019). https://doi.org/10.1007/s00234-019-02194-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-019-02194-0