Abstract
Purpose
To assess imaging, clinical, and pathological features of mesial temporal lobe epilepsy (mTLE) patients with amygdala enlargement (AE) in comparison with those with mesial temporal sclerosis (MTS).
Methods
Clinical, imaging, and pathologic features were retrospectively reviewed in 40 mTLE patients with postoperative follow-up (10 with AE and 30 with MTS). The volumes and signal intensity of the amygdala and hippocampus were assessed in 10 AE, 10 age- and sex-matched MTS patients, and 12 controls (HC).
Results
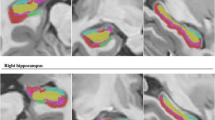
AE patients had a lower rate of concordant FDG PET (p < 0.05) and required more frequently intracerebral electrodes compared to MTS patients (p < 0.05). AE had larger ipsilateral amygdala (p < 0.0001) and hippocampus volumes (p < 0.0001) compared to MTS and to HC, with no significant differences for other brain structures. Normalized FLAIR signal was higher in the ipsilateral than contralateral amygdala in both AE and MTS (p < 0.001 and p < 0.05, respectively) and higher in the ipsilateral amygdala compared to HC (p < 0.05). In MTS, ADC in the ipsilateral amygdala (867 mm2/s) was higher compared to the contralateral one (804.8 × 10–6 mm2/s, p < 0.01), compared to HC (773 × 10–6 mm2/s, p < 0.01) and compared to the ipsilateral amygdala in AE (813.7 × 10–6 mm2/s, p < 0.05). AE patients had dysplasia (50%) or astrocytic gliosis (50%) of the amygdala extending to the hippocampus and temporal isocortex, and only 2/10 cases had pathologic findings of MTS.
Conclusion
AE patients have distinct imaging and pathologic features compared to MTS, and require more extensive preoperative workup. Recognition of AE may improve preoperative assessment in TLE surgical candidates.





Similar content being viewed by others
References
Mitsueda-Ono T, Ikeda A, Inouchi M, Takaya S, Matsumoto R, Hanakawa T, Sawamoto N, Mikuni N, Fukuyama H, Takahashi R (2011) Amygdalar enlargement in patients with temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 82(6):652–657
Beh SM, Cook MJ, D'Souza WJ (2016) Isolated amygdala enlargement in temporal lobe epilepsy: a systematic review. Epilepsy Behav 60:33–41
Minami N et al. (2014) Surgery for amygdala enlargement with mesial temporal lobe epilepsy: pathological findings and seizure outcome. J Neurol Neurosurg Psychiatry
Lv RJ, Sun ZR, Cui T, Guan HZ, Ren HT, Shao XQ (2014) Temporal lobe epilepsy with amygdala enlargement: a subtype of temporal lobe epilepsy. BMC Neurol 14:194
Kim DW, Lee SK, Chung CK, Koh YC, Choe G, Lim SD (2012) Clinical features and pathological characteristics of amygdala enlargement in mesial temporal lobe epilepsy. J Clin Neurosci 19(4):509–512
Bower SP, Vogrin SJ, Morris K, Cox I, Murphy M, Kilpatrick CJ, Cook MJ (2003) Amygdala volumetry in “imaging-negative” temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 74(9):1245–1249
Takaya S, Ikeda A, Mitsueda-Ono T, Matsumoto R, Inouchi M, Namiki C, Oishi N, Mikuni N, Ishizu K, Takahashi R, Fukuyama H (2014) Temporal lobe epilepsy with amygdala enlargement: a morphologic and functional study. J Neuroimaging 24(1):54–62
Malter MP et al. (2016) Suspected new-onset autoimmune temporal lobe epilepsy with amygdala enlargement. 57(9): p. 1485–94
Kimura Y, Sato N, Saito Y, Ito K, Kamiya K, Nakata Y, Watanabe M, Maikusa N, Matsuda H, Sugimoto H (2015) Temporal lobe epilepsy with unilateral amygdala enlargement: morphometric MR analysis with clinical and pathological study. J Neuroimaging 25(2):175–183
Reyes A, Thesen T, Kuzniecky R, Devinsky O, McDonald CR, Jackson GD, Vaughan DN, Blackmon K (2017) Amygdala enlargement: temporal lobe epilepsy subtype or nonspecific finding? Epilepsy Res 132:34–40
Luo W, A C, Albright J. The NeuroQuant Normative Database. Comparing Individual Brain Structures. I. Cortechs labs, Editor.: San Diego, CA USA
Engel J Jr (1993) Update on surgical treatment of the epilepsies. Summary of the second international Palm Desert conference on the surgical treatment of the epilepsies (1992). Neurology 43(8):1612–1617
Thom M, S S, Najm I (2008) Greenfield's neuropathology. p. 846–860
Muhlhofer W, Tan YL, Mueller SG, Knowlton R (2017) MRI-negative temporal lobe epilepsy-What do we know? Epilepsia 58(5):727–742
Téllez-Zenteno JF, Hernández Ronquillo L, Moien-Afshari F, Wiebe S (2010) Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res 89(2-3):310–8
Gloor P, Olivier A, Quesney LF, Andermann F, Horowitz S (1982) The role of the limbic system in experiential phenomena of temporal lobe epilepsy. Ann Neurol 12(2):129–44
Bendersky M, Rugilo C, Kochen S, Schuster G, Sica RE (2003) Magnetic resonance imaging identifies cytoarchitectonic subtypes of the normal human cerebral cortex. J Neurol Sci 211(1-2):75–80
Blumcke I et al (2011) The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc task force of the ILAE diagnostic methods commission. Epilepsia 52(1):158–174
Yu JT, Tan L (2008) Diffusion-weighted magnetic resonance imaging demonstrates parenchymal pathophysiological changes in epilepsy. Brain Res Rev 59(1):34–41
Azab M, Carone M, Ying SH, Yousem DM (2015) Mesial temporal sclerosis: accuracy of NeuroQuant versus Neuroradiologist. AJNR Am J Neuroradiol 36(8):1400–1406
Wehner T, LaPresto E, Tkach J, Liu P, Bingaman W, Prayson RA, Ruggieri P, Diehl B (2007) The value of interictal diffusion-weighted imaging in lateralizing temporal lobe epilepsy. Neurology 68(2):122–127
Yoo SY, Chang KH, Song IC, Han MH, Kwon BJ, Lee SH, Yu IK, Chun CK (2002) Apparent diffusion coefficient value of the hippocampus in patients with hippocampal sclerosis and in healthy volunteers. AJNR Am J Neuroradiol 23(5):809–812
Gloor P (1997) The temporal lobe and limbic system, in The Temporal Lobe and Limbic System. Oxford University Press, New York
Abel TJ, Woodroffe RW, Nourski KV, Moritani T, Capizzano AA, Kirby P, Kawasaki H, Howard M III, Werz MA (2018) Role of the temporal pole in temporal lobe epilepsy seizure networks: an intracranial electrode investigation. J Neurosurg 129(1):165–173
Reid AY, Staba RJ (2014) Limbic networks: clinical perspective. Int Rev Neurobiol 114:89–120
Gershen LD, Zanotti-Fregonara P, Dustin IH, Liow JS, Hirvonen J, Kreisl WC, Jenko KJ, Inati SK, Fujita M, Morse CL, Brouwer C, Hong JS, Pike VW, Zoghbi SS, Innis RB, Theodore WH (2015) Neuroinflammation in temporal lobe epilepsy measured using positron emission tomographic imaging of translocator protein. JAMA Neurol 72(8):882–888
Iyer A, Zurolo E, Spliet WGM, van Rijen PC, Baayen JC, Gorter JA, Aronica E (2010) Evaluation of the innate and adaptive immunity in type I and type II focal cortical dysplasias. Epilepsia 51(9):1763–1773
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
For this type of retrospective study formal consent is not required.
Rights and permissions
About this article
Cite this article
Capizzano, A.A., Kawasaki, H., Sainju, R.K. et al. Amygdala enlargement in mesial temporal lobe epilepsy: an alternative imaging presentation of limbic epilepsy. Neuroradiology 61, 119–127 (2019). https://doi.org/10.1007/s00234-018-2109-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-018-2109-y