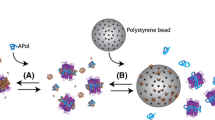
Abstract
Specific, tight-binding protein partners are valuable helpers to facilitate membrane protein (MP) crystallization, because they can i) stabilize the protein, ii) reduce its conformational heterogeneity, and iii) increase the polar surface from which well-ordered crystals can grow. The design and production of a new family of synthetic scaffolds (dubbed αReps, for “artificial alpha repeat protein”) have been recently described. The stabilization and immobilization of MPs in a functional state are an absolute prerequisite for the screening of binders that recognize specifically their native conformation. We present here a general procedure for the selection of αReps specific of any MP. It relies on the use of biotinylated amphipols, which act as a universal “Velcro” to stabilize, and immobilize MP targets onto streptavidin-coated solid supports, thus doing away with the need to tag the protein itself.






Similar content being viewed by others
Abbreviations
- 2XTY:
-
E. coli rich media
- A8-35:
-
A specific type of poly(acrylic acid)-based amphipol
- APol:
-
Amphipol
- BAPol:
-
Biotinylated A8-35
- BNAPol:
-
Biotinylated non-ionic amphipol
- BR:
-
Bacteriorhodopsin
- cmc:
-
Critical micellar concentration
- DDM:
-
Dodecyl-β-d-maltoside
- \(\overline{DP}_{n}\) :
-
Number-average degree of polymerization
- EDTA:
-
Ethylene diamine tetraacetic acid
- EM:
-
Electron microscopy
- His-tag:
-
Hexahistidine tag
- IPTG:
-
Isopropyl-β-d-1-thiogalactopyranoside
- MD:
-
Molecular dynamics
- \(\overline{M}_{n}\) :
-
Number-average molar mass
- MP:
-
Membrane protein
- MW:
-
Molecular weight
- MWCO:
-
MW cut-off
- NAPol:
-
Non-ionic amphipol
- NMR:
-
Nuclear magnetic resonance
- NTA:
-
Nitrilotriacetic acid
- OD600nm :
-
Optical density measured at 600 nm
- βOG:
-
n-octyl-β-d-glucopyranoside
- PBS:
-
Phosphate buffer saline
- PEG:
-
Polyethylene glycol
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SEC:
-
Size-exclusion chromatography
- TBS:
-
Tris-buffered saline
- TBST:
-
Tris-buffered saline supplemented with Tween 20 (w/v)
- Tris:
-
Tris(hydroxymethyl)aminomethane
References
Althoff T, Mills DJ, Popot J-L, Kühlbrandt W (2011) Assembly of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. EMBO J 30:4652–4664
Basit H, Sharma S, Van der Heyden A, Gondran C, Breyton C, Dumy P, Winnik FM, Labbé P (2012) Amphipol mediated surface immobilization of FhuA: a platform for label-free detection of the bacteriophage protein pb5. Chem Commun 48:6037–6039
Batchelor RH, Sarkez A, Cox WG, Johnson I (2007) Fluorometric assay for quantitation of biotin covalently attached to proteins and nucleic acids. Biotechniques 43:503–507
Bazzacco P, Billon-Denis E, Sharma KS, Catoire LJ, Mary S, Le Bon C, Point E, Banères J-L, Durand G, Zito F, Pucci B, Popot J-L (2012) Non-ionic homopolymeric amphipols: application to membrane protein folding, cell-free synthesis, and solution NMR. Biochemistry 51:1416–1430
Berry EA, Huang L-S, DeRose V (1991) Ubiquinol-cytochrome c oxidoreductase from higher plants. Isolation and characterization of the bc 1 complex from potato tuber mitochondria. J Biol Chem 266:9064–9077
Berry EA, Guergova-Kuras M, Huang L-S, Crofts AR (2000) Structure and function of cytochrome bc 1 complexes. Annu Rev Biochem 69:1005–1075
Binz HK, Stumpp MT, Forrer P, Amstutz P, Pluckthun A (2003) Designing repeat proteins: well-expressed, soluble and stable proteins from combinatorial libraries of consensus ankyrin repeat proteins. J Mol Biol 332:489–503
Boersma YL, Plückthun A (2011) DARPins and other repeat protein scaffolds: advances in engineering and applications. Curr Opin Biotechnol 22:849–857
Broutin I, Benabdelhak H, Moreel X, Lascombe MB, Lerouge D, Ducruix A (2005) Expression, purification, crystallization and preliminary X-ray studies of the outer membrane efflux proteins OprM and OprN from Pseudomonas aeruginosa. Acta Crystallogr Sect F Struct Biol Cryst Commun 61:315–318
Catoire LJ, Zoonens M, van Heijenoort C, Giusti F, Guittet E, Popot J-L (2010) Solution NMR mapping of water-accessible residues in the transmembrane β-barrel of OmpX. Eur Biophys J 39:623–630
Charvolin D, Perez JB, Rouviere F, Giusti F, Bazzacco P, Abdine A, Rappaport F, Martinez KL, Popot JL (2009) The use of amphipols as universal molecular adapters to immobilize membrane proteins onto solid supports. Proc Natl Acad Sci USA 106:405–410
Charvolin D, Picard M, Huang L-S, Berry EA, Popot J-L (2014) Solution behavior and crystallization of cytochrome bc 1 in the presence of amphipols. J Membr Biol. doi:10.1007/s00232-014-9694-4
Collins (2012) The Collins english dictionary. HarperCollins Publishers Limited, Glasgow
Cronan JE Jr (1990) Biotination of proteins in vivo. A post-translational modification to label, purify, and study proteins. J Biol Chem 265:10327–10333
Dahmane T, Rappaport F, Popot J-L (2013) Amphipol-assisted folding of bacteriorhodopsin in the presence and absence of lipids. Functional consequences. Eur Biophys J 42:85–101
Della Pia EA, Holm J, Lloret N, Le Bon C, Popot J-L, Zoonens M, Nygård J, Martinez KL (2014a) A step closer to membrane protein multiplexed nano-arrays using biotin-doped polypyrrole. ACS Nano 8:1844–1853
Della Pia EA, Westh Hansen R, Zoonens M, Martinez KL (2014b) Amphipols: a versatile toolbox suitable for applications of membrane proteins in synthetic biology. J Membr Biol. doi:10.1007/s00232-014-9663-y
Etzkorn M, Gelev V, Raschle T, Wagner G (2014) Use of amphipols for the NMR structural characterization of 7-TM receptors. J Membr Biol. doi:10.1007/s00232-014-9657-9
Giusti F, Rieger J, Catoire L, Qian S, Calabrese AN, Watkinson TG, Casiraghi M, Radford SE, Ashcroft AE, Popot J-L (2014) Synthesis, characterization and applications of a perdeuterated amphipol. J Membr Biol. doi:10.1007/s00232-014-9656-x
Gohon Y, Pavlov G, Timmins P, Tribet C, Popot JL, Ebel C (2004) Partial specific volume and solvent interactions of amphipol A8-35. Anal Biochem 334:318–334
Gohon Y, Giusti F, Prata C, Charvolin D, Timmins P, Ebel C, Tribet C, Popot JL (2006) Well-defined nanoparticles formed by hydrophobic assembly of a short and polydisperse random terpolymer, amphipol A8-35. Langmuir 22:1281–1290
Gohon Y, Dahmane T, Ruigrok RW, Schuck P, Charvolin D, Rappaport F, Timmins P, Engelman DM, Tribet C, Popot JL, Ebel C (2008) Bacteriorhodopsin/amphipol complexes: structural and functional properties. Biophys J 94:3523–3537
Guellouz A, Valerio-Lepiniec M, Urvoas A, Chevrel A, Graille M, Fourati-Kammoun Z, Desmadril M, van Tilbeurgh H, Minard P (2013) Selection of specific protein binders for pre-defined targets from an optimized library of artificial helicoidal repeat proteins (alphaRep). PLoS One 8:e71512
Huynh K, Cohen M, Moiseenkova-Bell V (2014) Application of amphipols for structure-functional analysis of TRP channels. J Membr Biol. doi:10.1007/s00232-014-9684-6
Koide S (2009) Engineering of recombinant crystallization chaperones. Curr Opin Struct Biol 19:449–457
Le Bon C, Della Pia EA, Giusti F, Lloret N, Zoonens M, Martinez KL, Popot J-L (2014a) Synthesis of an oligonucleotide-derivatized amphipol and its use to trap and immobilize membrane proteins. Nucleic Acids Res. doi:10.1093/nar/gku250
Le Bon C, Popot J-L, Giusti F (2014b) Labeling and functionalizing amphipols for biological applications. J Membr Biol. doi:10.1007/s00232-014-9655-y
Liao M, Cao E, Julius D, Cheng Y (2013) Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504:107–112
Liao M, Cao E, Julius D, Cheng Y (2014) Single particle electron cryo-microscopy of a mammalian ion channel. Curr Opin Struct Biol 27:1–7
Lieberman RL, Culver JA, Entzminger KC, Pai JC, Maynard JA (2011) Crystallization chaperone strategies for membrane proteins. Methods 55:293–302
Miroux B, Walker JE (1996) Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J Mol Biol 260:289–298
Mokhonov V, Mokhonova E, Yoshihara E, Masui R, Sakai M, Akama H, Nakae T (2005) Multidrug transporter MexB of Pseudomonas aeruginosa: overexpression, purification, and initial structural characterization. Protein Expr Purif 40:91–100
Muyldermans S, Baral TN, Retamozzo VC, De Baetselier P, De Genst E, Kinne J, Leonhardt H, Magez S, Nguyen VK, Revets H, Rothbauer U, Stijlemans B, Tillib S, Wernery U, Wyns L, Hassanzadeh-Ghassabeh G, Saerens D (2009) Camelid immunoglobulins and nanobody technology. Vet Immunol Immunopathol 128:178–183
Neutze R, Pebay-Peyroula E, Edman K, Royant A, Navarro J, Landau EM (2002) Bacteriorhodopsin: a high-resolution structural view of vectorial proton transport. Biochim Biophys Acta 1565:144–167
Ostermeier C, Iwata S, Ludwig B, Michel H (1995) Fv fragment-mediated crystallization of the membrane protein bacterial cytochrome c oxidase. Nature Struct Biol 2:842–846
Perlmutter JD, Drasler WJ, Xie W, Gao J, Popot J-L, Sachs JN (2011) All-atom and coarse-grained molecular dynamics simulations of a membrane protein stabilizing polymer. Langmuir 27:10523–10537
Perlmutter JD, Popot J-L, Sachs JN (2014) Molecular dynamics simulations of a membrane protein/amphipol complex. J Membr Biol. doi:10.1007/s00232-014-9690-8
Planchard N, Point E, Dahmane T, Giusti F, Renault M, Le Bon C, Durand G, Milon A, Guittet E, Zoonens M, Popot J-L, Catoire LJ (2014) The use of amphipols for solution NMR studies of membrane proteins: advantages and limitations as compared to other solubilizing media. J Membr Biol. doi:10.1007/s00232-014-9654-z
Popot J-L (2010) Amphipols, nanodiscs, and fluorinated surfactants: three non-conventional approaches to studying membrane proteins in aqueous solutions. Annu Rev Biochem 79:737–775
Popot J-L, Althoff T, Bagnard D, Banères J-L, Bazzacco P, Billon-Denis E, Catoire LJ, Champeil P, Charvolin D, Cocco MJ, Crémel G, Dahmane T, de la Maza LM, Ebel C, Gabel F, Giusti F, Gohon Y, Goormaghtigh E, Guittet E, Kleinschmidt JH, Kühlbrandt W, Le Bon C, Martinez KL, Picard M, Pucci B, Rappaport F, Sachs JN, Tribet C, van Heijenoort C, Wien F, Zito F, Zoonens M (2011) Amphipols from A to Z. Annu Rev Biophys 40:379–408
Pos KM (2009) Drug transport mechanism of the AcrB efflux pump. Biochim Biophys Acta 1794:782–793
Seeger MA, Zbinden R, Flutsch A, Gutte PG, Engeler S, Roschitzki-Voser H, Grutter MG (2013) Design, construction, and characterization of a second-generation DARPin library with reduced hydrophobicity. Protein Sci 22:1239–1257
Sennhauser G, Grutter MG (2008) Chaperone-assisted crystallography with DARPins. Structure 16:1443–1453
Sharma KS, Durand G, Gabel F, Bazzacco P, Le Bon C, Billon-Denis E, Catoire LJ, Popot JL, Ebel C, Pucci B (2012) Non-ionic amphiphilic homopolymers: synthesis, solution properties, and biochemical validation. Langmuir 28:4625–4639
Skerra A (2007) Alternative non-antibody scaffolds for molecular recognition. Curr Opin Biotechnol 18:295–304
Smith AL (1967) Preparation, properties, and conditions for assay of mitochondria: slaughterhouse material, small scale. Methods Enzymol 10:81–86
Soltes G, Barker H, Marmai K, Pun E, Yuen A, Wiersma EJ (2003) A new helper phage and phagemid vector system improves viral display of antibody Fab fragments and avoids propagation of insert-less virions. J Immunol Methods 274:233–244
Stroebel D, Sendra V, Cannella D, Helbig K, Nies D, Covès J (2007) Oligomeric behavior of the RND transporters CusA and AcrB in micellar solution of detergent. Biochim Biophys Acta 1768:1567–1573
Stumpp MT, Forrer P, Binz HK, Plückthun A (2003) Designing repeat proteins: modular leucine-rich repeat protein libraries based on the mammalian ribonuclease inhibitor family. J Mol Biol 332:471–487
Tribet C, Audebert R, Popot J-L (1996) Amphipols: polymers that keep membrane proteins soluble in aqueous solutions. Proc Natl Acad Sci USA 93:15047–15050
Tribet C, Audebert R, Popot J-L (1997) Stabilization of hydrophobic colloidal dispersions in water with amphiphilic polymers: application to integral membrane proteins. Langmuir 13:5570–5576
Tribet C, Diab C, Dahmane T, Zoonens M, Popot J-L, Winnik FM (2009) Thermodynamic characterization of the exchange of detergents and amphipols at the surfaces of integral membrane proteins. Langmuir 25:12623–12634
Urvoas A, Guellouz A, Valerio-Lepiniec M, Graille M, Durand D, Desravines DC, van Tilbeurgh H, Desmadril M, Minard P (2010) Design, production and molecular structure of a new family of artificial alpha-helicoidal repeat proteins (alphaRep) based on thermostable HEAT-like repeats. J Mol Biol 404:307–327
Zoonens M, Popot JL (2014) Amphipols for each season. J Membr Biol. doi:10.1007/s00232-014-9666-8
Zoonens M, Catoire LJ, Giusti F, Popot J-L (2005) NMR study of a membrane protein in detergent-free aqueous solution. Proc Natl Acad Sci USA 102:8893–8898
Zoonens M, Giusti F, Zito F, Popot JL (2007) Dynamics of membrane protein/amphipol association studied by Förster resonance energy transfer: implications for in vitro studies of amphipol-stabilized membrane proteins. Biochemistry 46:10392–10404
Zoonens M, Zito F, Martinez KL, Popot J-L (2014) Amphipols: a general introduction and some protocols. In: Mus-Veteau I (ed) Membrane protein production for structural analysis. Springer, New York, in the press
Acknowledgments
Particular thanks are due to J.D. Perlmutter and J.N. Sachs for communication of a molecular dynamics model of an A8-35 particle. This research was supported by ANR-2010-BLAN-1535, by the French Centre National de la Recherche Scientifique (CNRS), by Université Paris-5 Paris Descartes, by Université Paris-7 Denis Diderot, and by grant “DYNAMO”, ANR-11-LABX-0011-01 from the French “Initiative d’Excellence” program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferrandez, Y., Dezi, M., Bosco, M. et al. Amphipol-Mediated Screening of Molecular Orthoses Specific for Membrane Protein Targets. J Membrane Biol 247, 925–940 (2014). https://doi.org/10.1007/s00232-014-9707-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-014-9707-3