Abstract
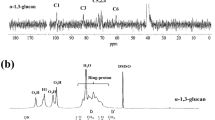
Amphipols are short amphipathic polymers that can substitute for detergents at the hydrophobic surface of membrane proteins (MPs), keeping them soluble in the absence of detergents while stabilizing them. The most widely used amphipol, known as A8-35, is comprised of a polyacrylic acid (PAA) main chain grafted with octylamine and isopropylamine. Among its many applications, A8-35 has proven particularly useful for solution-state NMR studies of MPs, for which it can be desirable to eliminate signals originating from the protons of the surfactant. In the present work, we describe the synthesis and properties of perdeuterated A8-35 (perDAPol). Perdeuterated PAA was obtained by radical polymerization of deuterated acrylic acid. It was subsequently grafted with deuterated amines, yielding perDAPol. The number-average molar mass of hydrogenated and perDAPol, ~4 and ~5 kDa, respectively, was deduced from that of their PAA precursors, determined by size exclusion chromatography in tetrahydrofuran following permethylation. Electrospray ionization–ion mobility spectrometry–mass spectrometry measurements show the molar mass and distribution of the two APols to be very similar. Upon neutron scattering, the contrast match point of perDAPol is found to be ~120 % D2O. In 1H-1H nuclear overhauser effect NMR spectra, its contribution is reduced to ~6 % of that of hydrogenated A8-35, making it suitable for extended uses in NMR spectroscopy. PerDAPol ought to also be of use for inelastic neutron scattering studies of the dynamics of APol-trapped MPs, as well as small-angle neutron scattering and analytical ultracentrifugation.








Similar content being viewed by others
Abbreviations
- 1D, 2D, 3D:
-
One-, two- and three-dimensional, respectively
- A8-35:
-
Sodium poly(acrylate)-based amphipol with a weight-average molar mass close to 8 kDa and containing 35 % of free carboxylate
- A8-75:
-
Sodium poly(acrylate)-based amphipol with a weight-average molar mass close to 8 kDa and containing 75 % of free carboxylate
- AA:
-
Acrylic acid
- AAd4 :
-
Acrylic acid-d4
- AIBN:
-
2,2′-Azoisobutyronitrile
- APol:
-
Amphipol
- AUC:
-
Analytical ultracentrifugation
- CMP:
-
Neutron scattering contrast match point
- C t :
-
Transfer constant
- DAPol:
-
A8-35 with perdeuterated side chains
- DCI:
-
Dicyclohexylcarbodiimide
- DCU:
-
N,N-Dicyclohexylurea
- \(\overline{{DP_{n} }}\) :
-
Average degree of polymerization in number
- dV:
-
Differential viscometry
- Đ:
-
Molar mass dispersity
- ESI:
-
Electrospray ionization
- HAPol:
-
Hydrogenated A8-35
- HSQC:
-
Heteronuclear single quantum correlation
- HOBt:
-
1-N-Hydroxybenzotriazole
- IMS:
-
Ion mobility spectrometry
- INS:
-
Inelastic neutron scattering
- LTB4 :
-
Leukotriene B4
- \(\overline{{M_{n} }}\) :
-
Number-average molar mass
- mQ water:
-
Water purified on a A10 advantage millipore system
- MS:
-
Mass spectrometry
- \(\overline{{M_{w} }}\) :
-
Weight-average molar mass
- NAPol:
-
Non-ionic amphipol
- NMP:
-
N-Methylpyrrolidone
- NOE:
-
Nuclear Overhauser effect
- NOESY:
-
NOE spectroscopy
- PAA:
-
Poly(acrylic acid)
- perDAPol:
-
Perdeuterated A8-35
- PEO:
-
Poly(ethylene oxide)
- PS:
-
Polystyrene
- RI:
-
Refractive index
- R S :
-
Stokes radius
- SANS:
-
Small-angle neutron scattering
- SAPol:
-
Sulfonated amphipol derived from A8-75
- SEC:
-
Size-exclusion chromatography
- TA:
-
Transfer agent
- TGA:
-
Thioglycolic acid
- THF:
-
Tetrahydrofuran
- TMSCHN2 :
-
Trimethylsilyldiazomethane
- WHH:
-
Width at half-height
References
Banères J-L, Popot J-L, Mouillac B (2011) New advances in production and functional folding of G protein-coupled receptors. Trends Biotechnol 29:314–322
Bazzacco P, Billon-Denis E, Sharma KS, Catoire LJ, Mary S, Le Bon C, Point E, Banères J-L, Durand G, Zito F, Pucci B, Popot J-L (2012) Non-ionic homopolymeric amphipols: application to membrane protein folding, cell-free synthesis, and solution NMR. Biochemistry 51:1416–1430
Berry KD, Bailey KM, Beal J, Diawara Y, Funk L, Steve Hicks J, Jones AB, Littrell KC, Pingali SV, Summers PR, Urban VS, Vandergriff DH, Johnson NH, Bradley BJ (2012) Characterization of the neutron detector upgrade to the GP-SANS and Bio-SANS instruments at HFIR. Nucl Instr Meth Phys Res A 693:179–185
Catoire LJ, Zoonens M, van Heijenoort C, Giusti F, Popot J-L, Guittet E (2009) Inter- and intramolecular contacts in a membrane protein/surfactant complex observed by heteronuclear dipole-to-dipole cross-relaxation. J Magn Res 197:91–95
Catoire LJ, Damian M, Giusti F, Martin A, van Heijenoort C, Popot J-L, Guittet E, Banères J-L (2010a) Structure of a GPCR ligand in its receptor-bound state: leukotriene B4 adopts a highly constrained conformation when associated to human BLT2. J Am Chem Soc 132:9049–9057
Catoire LJ, Zoonens M, van Heijenoort C, Giusti F, Guittet E, Popot J-L (2010b) Solution NMR mapping of water-accessible residues in the transmembrane β-barrel of OmpX. Eur Biophys J 39:623–630
Catoire LJ, Damian M, Baaden M, Guittet E, Banères J-L (2011) Electrostatically-driven fast association and perdeuteration allow detection of transferred cross-relaxation for G protein-coupled receptor ligands with equilibrium dissociation constants in the high-to-low nanomolar range. J Biomol NMR 50:191–195
Catoire LJ, Warnet XL, Warschawski DE (2014) Micelles, bicelles, amphipols, nanodiscs, liposomes or intact cells: The hitch-hiker guide to membrane protein study by NMR. In: Mus-Veteau I (ed) Membrane protein production for structural analysis. Springer, Berlin (in press)
Charvolin D, Perez J-B, Rouvière F, Giusti F, Bazzacco P, Abdine A, Rappaport F, Martinez KL, Popot J-L (2009) The use of amphipols as universal molecular adapters to immobilize membrane proteins onto solid supports. Proc Natl Acad Sci USA 106:405–410
Couvreur L, Lefay C, Belleney J, Charleux B, Guerret O, Magnet S (2003) First nitroxide-mediated controlled free-radical polymerization of acrylic acid. Macromolecules 36:8260–8267
Czerski L, Sanders CR (2000) Functionality of a membrane protein in bicelles. Anal Biochem 284:327–333
Dahmane T, Damian M, Mary S, Popot J-L, Banères J-L (2009) Amphipol-assisted in vitro folding of G protein-coupled receptors. Biochemistry 48:6516–6521
Dahmane T, Giusti F, Catoire LJ, Popot J-L (2011) Sulfonated amphipols: synthesis, properties and applications. Biopolymers 95:811–823
Diab C, Tribet C, Gohon Y, Popot J-L, Winnik FM (2007a) Complexation of integral membrane proteins by phosphorylcholine-based amphipols. Biochim Biophys Acta 1768:2737–2747
Diab C, Winnik FM, Tribet C (2007b) Enthalpy of interaction and binding isotherms of non-ionic surfactants onto micellar amphiphilic polymers (amphipols). Langmuir 23:3025–3035
Elter, S, Raschle, T, Arens, S, Gelev, V, Etzkorn, M, Wagner, G (2014). The use of amphipols for NMR structural characterization of 7-TM proteins (submitted for publication)
Etzkorn M, Raschle T, Hagn F, Gelev V, Rice AJ, Walz T, Wagner G (2013) Cell-free expressed bacteriorhodopsin in different soluble membrane mimetics: biophysical properties and NMR accessibility. Structure 21:394–401
Etzkorn, M, Zoonens, M, Catoire, LJ, Popot, J-L, Hiller, S (2014). How amphipols embed membrane proteins: Global solvent accessibility and interaction with a flexible protein terminus. J Membr Biol (in press)
Fernandez, A, Le Bon, C, Baumlin, N, Giusti, F, Crémel, G, Popot, J-L, Bagnard, D (2014) In vivo characterization of the biodistribution profile of amphipols (submitted for publication)
Giusti F, Popot J-L, Tribet C (2012) Well-defined critical association concentration and rapid adsorption at the air/water interface of a short amphiphilic polymer, amphipol A8-35: a study by Förster resonance energy transfer and dynamic surface tension measurements. Langmuir 28:10372–10380
Giusti, F, Kessler, P, Westh Hansen, R, Della Pia, EA, Le Bon, C, Mourier, G, Popot, J-L, Martinez, KL, Zoonens, M (2014). Synthesis of polyhistidine- or imidazole-bearing amphipols and their use for immobilized metal affinity chromatography and surface plasmon resonance studies of membrane proteins (in preparation)
Glück JM, Wittlich M, Feuerstein S, Hoffmann S, Willbold D, Koenig BW (2009) Integral membrane proteins in nanodiscs can be studied by solution NMR spectroscopy. J Am Chem Soc 131:12060–12061
Gohon Y, Pavlov G, Timmins P, Tribet C, Popot J-L, Ebel C (2004) Partial specific volume and solvent interactions of amphipol A8-35. Anal Biochem 334:318–334
Gohon Y, Giusti F, Prata C, Charvolin D, Timmins P, Ebel C, Tribet C, Popot J-L (2006) Well-defined nanoparticles formed by hydrophobic assembly of a short and polydisperse random terpolymer, amphipol A8-35. Langmuir 22:1281–1290
Gohon Y, Dahmane T, Ruigrok R, Schuck P, Charvolin D, Rappaport F, Timmins P, Engelman DM, Tribet C, Popot J-L, Ebel C (2008) Bacteriorhodopsin/amphipol complexes: structural and functional properties. Biophys J 94:3523–3537
Guinier A, Fournet G (1955) Small-angle scattering of X-rays. John Wiley and sons, New York
Hagn F, Etzkorn M, Raschle T, Wagner G (2013) Optimized phospholipid bilayer nanodiscs facilitate high-resolution structure determination of membrane proteins. J Am Chem Soc 135:1919–1925
Hamberg M, Svensson J, Samuelsson B (1974) Prostaglandin endoperoxides. A new concept concerning the mode of action and release of prostaglandins. Proc Natl Acad Sci USA 71:3824–3828
Hernandez H, Robinson CV (2007) Determining the stoichiometry and interactions of macromolecular assemblies from mass spectrometry. Nat Protocols 2:715–726
Huynh, KW, Cohen, MR, Moiseenkova-Bell, VY (2014). Application of amphipols for structure-functional analysis of TRP channels (submitted for publication)
Kang CB, Li Q (2011) Solution NMR study of integral membrane proteins. Curr Opin Struct Biol 15:560–569
Kanu AB, Dwivedi P, Tam M, Matz L, Hill HH Jr (2008) Ion mobility: mass spectrometry. J Mass Spectrom 43:1–22
Kay LE, Ikura M, Tschudin R, Bax A (1990) Three-dimensional triple resonance NMR spectroscopy of isotopically enriched proteins. J Magn Reson 89:496–514
Kline SR (2006) Reduction and analysis of SANS and USANS data using IGOR Pro. J Appl Crystall 39:895–900
Le Bon, C, Della Pia, EA, Giusti, F, Lloret, N, Zoonens, M, Martinez, KL, Popot, J-L (2014a). Synthesis of an oligonucleotide-derivatized amphipol and its use to trap and immobilize membrane proteins. Nucleic Acids Res (in press)
Le Bon, C, Popot, J-L, Giusti, F (2014b). Labeling and functionalizing amphipols for biological applications. J Membr Biol (in press)
Leney AC, McMorran LM, Radford SE, Ashcroft AE (2012) Amphipathic polymers enable the study of functional membrane proteins in the gas phase. Anal Chem 84:9841–9847
Loubat C, Javidan A, Boutevin B (2000) Etude de la télomérisation de l’acide acrylique par les mercaptans. 1. Télomérisation de l’acide acrylique par l’acide thioglycolique. Influence de la nature du solvant sur la valeur de la constante de transfert et de k p/√k te. Macromol Chem Phys 201:2845–2852
Lynn GW, Heller W, Urban V, Wignall GD, Weiss K, Myles DAA (2006) A dedicated facility for neutron structural biology at Oak Ridge National Laboratory. Physica B 385–386:880–882
Mazhab-Jafari MT, Marshall CB, Stathopulos PB, Kobashigawa Y, Stambolic V, Kay LE, Inagaki F, Ikura M (2013) Membrane-dependent modulation of the mTOR activator Rheb: NMR observations of a GTPase tethered to a lipid-bilayer nanodisc. J Am Chem Soc 135:3367–3370
McGregor C-L, Chen L, Pomroy NC, Hwang P, Go S, Chakrabartty A, Privé GG (2003) Lipopeptide detergents designed for the structural study of membrane proteins. Nat Biotechnol 21:171–176
Opačić, M, Giusti, F, Broos, J, Popot, J-L (2014). Isolation of Escherichia coli mannitol permease, EIImtl, trapped in amphipol A8-35 (submitted for publication)
Pavia AA, Pucci B, Riess JG, Zarrif L (1992) New perfluoroalkyl telomeric non-ionic surfactants: synthesis, physicochemical and biological properties. Makromol Chem 193:2505–2517
Perlmutter JD, Drasler WJ, Xie W, Gao J, Popot J-L, Sachs JN (2011) All-atom and coarse-grained molecular dynamics simulations of a membrane protein stabilizing polymer. Langmuir 27:10523–10537
Perlmutter, JD, Popot, J-L, Sachs, JN (2014). Molecular dynamics simulations of a membrane protein/amphipol complex (submitted for publication)
Picard M, Dahmane T, Garrigos M, Gauron C, Giusti F, le Maire M, Popot J-L, Champeil P (2006) Protective and inhibitory effects of various types of amphipols on the Ca2+-ATPase from sarcoplasmic reticulum: a comparative study. Biochemistry 45:1861–1869
Planchard, N, Point, E, Dahmane, T, Giusti, F, Renault, M, Le Bon, C, Durand, G, Milon, A, Guittet, E, Zoonens, M, Popot, J-L, Catoire, LJ (2014). The use of amphipols for solution NMR studies of membrane proteins: advantages and limitations as compared to other solubilizing media. J Membr Biol (in press)
Plevin, MJ, Boisbouvier J (2012). Isotope-labelling of methyl groups for NMR studies of large proteins. In M Clore and J Potts (eds) Recent developments in biomolecular NMR, Royal Society of Chemistry pp 1–24
Pocanschi C, Popot J-L, Kleinschmidt JH (2013) Folding and stability of outer membrane protein A (OmpA) from Escherichia coli in an amphipathic polymer, amphipol A8-35. Eur Biophys J 42:103–118
Popot J-L (2010) Amphipols, nanodiscs, and fluorinated surfactants: three non-conventional approaches to studying membrane proteins in aqueous solutions. Annu Rev Biochem 79:737–775
Popot J-L, Althoff T, Bagnard D, Banères J-L, Bazzacco P, Billon-Denis E, Catoire LJ, Champeil P, Charvolin D, Cocco MJ, Crémel G, Dahmane T, de la Maza LM, Ebel C, Gabel F, Giusti F, Gohon Y, Goormaghtigh E, Guittet E, Kleinschmidt JH, Kühlbrandt W, Le Bon C, Martinez KL, Picard M, Pucci B, Rappaport F, Sachs JN, Tribet C, van Heijenoort C, Wien F, Zito F, Zoonens M (2011) Amphipols from A to Z. Annu Rev Biophys 40:379–408
Presser A, Hüfner A (2004) Trimethylsilyldiazomethane. A mild and efficient reagent for the methylation of carboxylic acids and alcohols in natural products. Monatsch Chem 135:1015–1022
Privé G (2009) Lipopeptide detergents for membrane protein studies. Curr Opin Struct Biol 19:1–7
Raschle T, Hiller S, Yu TY, Rice AJ, Walz T, Wagner G (2009) Structural and functional characterization of the integral membrane protein VDAC-1 in lipid bilayer nanodiscs. J Am Chem Soc 131:17777–17779
Raschle T, Hiller S, Etzkorn M, Wagner G (2010) Nonmicellar systems for solution NMR spectroscopy of membrane proteins. Curr Opin Struct Biol 20:471–479
Renault M (2008) Etudes structurales et dynamiques de la protéine membranaire KpOmpA par RMN en phase liquide et solide, Ph. D. Thesis, Université Paul Sabatier, Toulouse
Salzmann M, Wider G, Pervushin K, Wüthrich K (1999) Improved sensitivity and coherence selection for [15N,1H]-TROSY elements in triple resonance experiments. J Biomol NMR 15:181–184
Sanders CR II, Landis GC (1995) Reconstitution of membrane proteins into lipid-rich bilayered mixed micelles for NMR studies. Biochemistry 34:4030–4040
Sanders CR, Prosser RS (1998) Bicelles: a model membrane system for all seasons? Structure 6:1227–1234
Shenkarev ZO, Lyukmanova EN, Paramonov AS, Shingarova LN, Chupin VV, Kirpich-ni-kov MP, Blommers MJ, Arseniev AS (2010) Lipid-protein nanodiscs as reference medium in detergent screening for high-resolution NMR studies of integral membrane proteins. J Am Chem Soc 132:5628–5629
Sprangers R, Velyvis A, Kay LE (2007) Solution NMR of supramolecular complexes: providing new insights into function. Nat Meth 4:697–703
Sverzhinsky, A, Qian, S, Yang, L, Allaire, M, Moraes, I, Ma, D, Chung, JW, Zoonens, M, Popot, J-L, Coulton, JW (2014). Amphipol-trapped ExbB—ExbD membrane protein complex from Escherichia coli: A biochemical and structural case study (submitted for publication)
Tehei, M, Giusti, F, Popot, J-L, Zaccai, G (2014). Thermal fluctuations in amphipol A8-35 measured by neutron scattering (submitted for publication)
Tribet C, Audebert R, Popot J-L (1996) Amphipols: polymers that keep membrane proteins soluble in aqueous solutions. Proc Natl Acad Sci USA 93:15047–15050
Tribet C, Diab C, Dahmane T, Zoonens M, Popot J-L, Winnik FM (2009) Thermodynamic characterization of the exchange of detergents and amphipols at the surfaces of integral membrane proteins. Langmuir 25:12623–12634
Tzitzilonis C, Eichmann C, Maslennikov I, Choe S, Riek R (2013) Detergent/nanodisc screening for high-resolution NMR studies of an integral membrane protein containing a cytoplasmic domain. PLoS ONE 8:e54378
Velasquez E, Pembouong G, Rieger J, Stoffelbach F, Boyron O, Charleux B, D’Agosto F, Lansalot M, Dufils P-E, Vinas J (2013) Poly(vinylidene chloride)-based amphiphilic block copolymers. Macromolecules 46:664–673
Warschawski DE, Arnold AA, Beaugrand M, Gravel A, Chartrand E, Marcotte I (2011) Choosing membrane mimetics for NMR structural studies of transmembrane proteins. Biochim Biophys Acta 1808:1957–1974
Weidner SM, Trimpin S (2010) Mass spectrometry of synthetic polymers. Anal Chem 82:4811–4829
Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T (2000) A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J Exp Med 192:421–432
Zaccai G, Jacrot B (1983) Small-angle neutron scattering. Annu Rev Biophys Bioeng 12:139–157
Zoonens, M, Popot, J-L (2014). Amphipols for each season (submitted for publication)
Zoonens M, Catoire LJ, Giusti F, Popot J-L (2005) NMR study of a membrane protein in detergent-free aqueous solution. Proc Natl Acad Sci USA 102:8893–8898
Zoonens M, Giusti F, Zito F, Popot J-L (2007) Dynamics of membrane protein/amphipol association studied by Förster resonance energy transfer. Implications for in vitro studies of amphipol-stabilized membrane proteins. Biochemistry 46:10392–10404
Acknowledgments
Particular thanks are due to Alain Fradet (UPMC - CNRS, IPCM) for his support and his comments on the manuscript, to Gaëlle Pembouong and Marion Chenal (same laboratory) for assistance with SEC analyses, to Christophe Tribet (Ecole Normale Supérieure, Paris) for his kind help at interpreting the results of the SEC experiments and to the Biotechnology and Biological Sciences Research Council of the UK for funding for the Synapt HDMS mass spectrometer (BB/E012558/1), ANC (BB/K000659/1) and TGW (BB/K501827/1). This work was supported by the French Centre National de la Recherche Scientifique (CNRS), by Université Paris-7 Denis Diderot, by grant “DYNAMO,” ANR-11-LABX-0011-01 from the French “Initiative d’Excellence” program, by the Office of Biological and Environmental Research, US Department of Energy (Bio-SANS, operated by ORNL’s Center for Structural Molecular Biology) and the Scientific User Facilities Division, Office of Basic Energy Sciences, US Department of Energy (ORNL’s High Flux Isotope Reactor).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giusti, F., Rieger, J., Catoire, L.J. et al. Synthesis, Characterization and Applications of a Perdeuterated Amphipol. J Membrane Biol 247, 909–924 (2014). https://doi.org/10.1007/s00232-014-9656-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-014-9656-x