Abstract
Purpose
This study aimed to investigate the effects of clotrimazole on the pharmacokinetics of tacrolimus in Japanese patients with heart transplants with different CYP3A5 genotypes.
Methods
Twenty-six patients who underwent heart transplantation between June 2012 and July 2017 were enrolled in this retrospective study. The CYP3A5 (rs776746; CYP3A5*3) genotype was determined after monitoring and analysing tacrolimus blood concentrations. The pharmacokinetic profile of tacrolimus was examined before and after the discontinuation of clotrimazole and in patients with different CYP3A5 genotypes.
Results
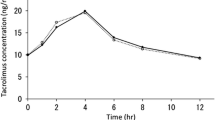
The CYP3A5*1/*1, *1/*3 and *3/*3 genotypes were detected in 2, 8 and 16 patients, respectively. After clotrimazole was discontinued, the CYP3A5 expresser (CYP3A5*1/*1 or *1/*3) group had a 3.3-fold median increase in apparent oral clearance of tacrolimus (0.27 vs. 0.89 L/h/kg, P = 0.002) compared with the CYP3A5 non-expresser (CYP3A5*3/*3) group with a 2.2-fold median increase (0.18 vs. 0.39 L/h/kg, P < 0.0001). Significant correlations were observed between C0 and area under the concentration–time curve (AUC0–12) of tacrolimus after the discontinuation of clotrimazole in the CYP3A5 expresser and non-expresser groups, respectively (R2 = 0.49 and 0.42, all P < 0.05), but not before the discontinuation of clotrimazole.
Conclusion
The effects of clotrimazole on tacrolimus pharmacokinetics in the CYP3A5 expresser patients were significantly greater than those in the CYP3A5 non-expresser patients. In addition, clotrimazole disturbed the correlation between C0 and AUC0–12 of tacrolimus. Careful dose adjustment of tacrolimus based on CYP3A5 genotypes may be beneficial for the patients with heart transplants who are concomitantly treated with clotrimazole.

Similar content being viewed by others
References
Margreiter R (2002) Efficacy and safety of tacrolimus compared with ciclosporin microemulsion in renal transplantation: a randomised multicentre study. Lancet 359:741–746
Böttiger Y, Brattström C, Tydén G, Säwe J, Groth C (1999) Tacrolimus whole blood concentrations correlate closely to side-effects in renal transplant recipients. Br J Clin Pharmacol 48(3):445–448
Dai Y, Hebert M, Isoherranen N, Davis C, Marsh C, Shen D, Thummel K (2006) Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab Dispos 34(5):836–847
Staatz C, Taylor P, Tett S (2001) Low tacrolimus concentrations and increased risk of early acute rejection in adult renal transplantation. Nephrology Dialysis Transplantation 16(9):1905–1909
Glotzbecker B, Duncan C, Alyea E 3rd, Campbell B, Soiffer R (2012) Important drug interactions in hematopoietic stem cell transplantation: what every physician should know. Biol Blood Marrow Transplant 18(7):989–1006
Sattler M, Guengerich F, Yun C, Christians U, Sewing K (1992) Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab Dispos 20(5):753–761
Iwasaki K (2007) Metabolism of tacrolimus (FK506) and recent topics in clinical pharmacokinetics. Drug metabolism Pharmacokinetics 22(5):328–335
Uesugi M, Masuda S, Katsura T, Oike F, Takada Y, Inui K (2006) Effect of intestinal CYP3A5 on postoperative tacrolimus trough levels in living-donor liver transplant recipients. Pharmacogenet Genomics 16(2):119–127
Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins P, Daly A, Wrighton S, Hall S, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski M, Schuetz E (2001) Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet 27(4):383–391
Hiratsuka M, Takekuma Y, Endo N, Narahara K, Hamdy SI, Kishikawa Y, Matsuura M, Agatsuma Y, Inoue T, Mizugaki M (2002) Allele and genotype frequencies of CYP2B6 and CYP3A5 in the Japanese population. Eur J Clin Pharmacol 58(6):417–421
Staatz C, Goodman L, Tett S (2010) Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part I. Clin Pharmacokinet 49(3):141–175
Saad A, DePestel D, Carver P (2006) Factors influencing the magnitude and clinical significance of drug interactions between azole antifungals and select immunosuppressants. Pharmacotherapy 26(12):1730–1744
Nara M, Takahashi N, Miura M, Niioka T, Kagaya H, Fujishima N, Saitoh H, Kameoka Y, Tagawa H, Hirokawa M, Sawada K (2013) Effect of itraconazole on the concentrations of tacrolimus and cyclosporine in the blood of patients receiving allogeneic hematopoietic stem cell transplants. Eur J Clin Pharmacol 69(6):1321–1329
Togashi M, Niioka T, Komatsuda A, Nara M, Okuyama S, Omokawa A, Abumiya M, Wakui H, Takahashi N, Miura M (2015) Effect of CYP3A5 and ABCB1 polymorphisms on the interaction between tacrolimus and itraconazole in patients with connective tissue disease. Eur J Clin Pharmacol 71(9):1091–1097
Lalan S, Abdel-Rahman S, Gaedigk A, Leeder JS, Warady BA, Dai H, Blowey D (2014) Effect of CYP3A5 genotype, steroids, and azoles on tacrolimus in a pediatric renal transplant population. Pediatr Nephrol 29(10):2039–2049
Yamashita T, Fujishima N, Miura M, Niioka T, Abumiya M, Shinohara Y, Ubukawa K, Nara M, Fujishima M, Kameoka Y, Tagawa H, Hirokawa M, Takahashi N (2016) Effects of CYP3A5 polymorphism on the pharmacokinetics of a once-daily modified-release tacrolimus formulation and acute kidney injury in hematopoietic stem cell transplantation. Cancer Chemother Pharmacol 78(1):111–118
Gombert M, duBouchet L, Aulicino T, Butt K (1987) A comparative trial of clotrimazole troches and oral nystatin suspension in recipients of renal transplants. Use in prophylaxis of oropharyngeal candidiasis. JAMA 258(18):2553–2555
Owens N, Nightingale C, Schweizer R, Schauer P, Dekker P, Quintiliani R (1984) Prophylaxis of oral candidiasis with clotrimazole troches. Arch Intern Med 144(2):290–293
El-Asmar J, Gonzalez R, Bookout R, Mishra A, Kharfan-Dabaja MA (2016) Clotrimazole troches induce supratherapeutic blood levels of sirolimus and tacrolimus in an allogeneic hematopoietic cell-transplant recipient resulting in acute kidney injury. Hematol Oncol Stem Cell Ther 9(4):157–161
Mieles L, Venkataramanan R, Yokoyama I, Warty V, Starzl T (1991) Interaction between FK506 and clotrimazole in a liver transplant recipient. Transplantation 52(6):1086–1087
Choy M (2010) Tacrolimus interaction with clotrimazole: a concise case report and literature review. P & T : a peer-Reviewed J Formulary Management 35(10):568–569
Vasquez E, Pollak R, Benedetti E (2001) Clotrimazole increases tacrolimus blood levels: a drug interaction in kidney transplant patients. Clin Transpl 15(2):95–99
Vasquez E, Shin G, Sifontis N, Benedetti E (2005) Concomitant clotrimazole therapy more than doubles the relative oral bioavailability of tacrolimus. Ther Drug Monit 27(5):587–591
Viesselmann CW, Descourouez JL, Jorgenson MR, Radke NA, Odorico JS (2016) Clinically significant drug interaction between clotrimazole and tacrolimus in pancreas transplant recipients and associated risk of allograft rejection. Pharmacotherapy 36(3):335–341
Cangemi G, Barco S, Bonifazio P, Maffia A, Agazzi A, Melioli G (2013) Comparison of antibody-conjugated magnetic immunoassay and liquid chromatography-tandem mass spectrometry for the measurement of cyclosporine and tacrolimus in whole blood. Int J Immunopathol Pharmacol 26(2):419–426
Pfeffer M (1984) Estimation of mean residence time from data obtained when multiple-dosing steady state has been reached. J Pharm Sci 73(6):854–856
Anglicheau D, Flamant M, Schlageter M, Martinez F, Cassinat B, Beaune P, Legendre C, Thervet E (2003) Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrology Dialysis Transplantation 18(11):2409–2414
Schiff J, Cole E, Cantarovich M (2007) Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol 2(2):374–384
Shord SS, Chan LN, Camp JR, Vasquez EM, Jeong HY, Molokie RE, Baum CL, Xie H (2010) Effects of oral clotrimazole troches on the pharmacokinetics of oral and intravenous midazolam. Br J Clin Pharmacol 69(2):160–166
Samaranayake L, Keung Leung W, Jin L (2009) Oral mucosal fungal infections. Periodontology 49:39–59
Liu M, Chen M, Yang Z (2017) Design of amphotericin B oral formulation for antifungal therapy. Drug Deliv 24(1):1–9
Paterson D, Singh N (1997) Interactions between tacrolimus and antimicrobial agents. Clinical Infectious Diseases: an official publication Infectious Diseases Soc Am 25(6):1430–1440
Ohshima T, Miyakawa Y, Watanabe T, Ohyama K (2003) Effect of amphotericin B dilution with various beverages on the survival of Candida albicans cells. Kansenshōgaku zasshi J Japanese Assoc Infectious Diseases 77(1):29–33
Lyu X, Zhao C, Yan ZM, Hua H (2016) Efficacy of nystatin for the treatment of oral candidiasis: a systematic review and meta-analysis. Drug Des Devel Ther 10:1161–1171
Nakatani T, Fukushima N, Ono M, Saiki Y, Matsuda H, Nunoda S, Sawa Y, Isobe M (2016) The Registry Report of Heart Transplantation in Japan (1999-2014). Circ J 80(1):44–50
Shi WL, Tang HL, Zhai SD (2015) Effects of the CYP3A4*1B genetic polymorphism on the pharmacokinetics of tacrolimus in adult renal transplant recipients: a meta-analysis. PLoS One 10(6):e0127995
Santoro A, Felipe C, Tedesco-Silva H, Medina-Pestana J, Struchiner C, Ojopi E, Suarez-Kurtz G (2011) Pharmacogenetics of calcineurin inhibitors in Brazilian renal transplant patients. Pharmacogenomics 12(9):1293–1303
Lamba J, Lin Y, Schuetz E, Thummel K (2002) Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev 54(10):1271–1294
Balram C, Zhou Q, Cheung Y, Lee E (2003) CYP3A5*3 and *6 single nucleotide polymorphisms in three distinct Asian populations. Eur J Clin Pharmacol 59(2):123–126
Acknowledgements
We would like to thank all the patients who participated in this study.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Participated in research design: Uno, Wada, Matsuda, Terada and Takada
Conducted experiments and clinical study: Uno, Wada, Matsuda, Terada and Takada
Performed data analysis: Uno, Wada, Kawase and Takada
Wrote or contributed to the writing of the manuscript: Uno, Wada, Terakawa, Oita, Kawase, Yokoyama, Hosomi and Takada
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was approved by the local ethic committee of the National Cerebral and Cardiovascular Center.
Informed consent
An informed consent was obtained from all individual participants included in the study.
Additional information
This study was conducted in the Department of Pharmacy, National Cerebral and Cardiovascular Center, 5-7-1 Fujishiro-dai, Suita, Osaka, Japan.
Electronic supplementary material
ESM 1
(DOCX 107 kb)
Rights and permissions
About this article
Cite this article
Uno, T., Wada, K., Matsuda, S. et al. Effects of clotrimazole on tacrolimus pharmacokinetics in patients with heart transplants with different CYP3A5 genotypes. Eur J Clin Pharmacol 75, 67–75 (2019). https://doi.org/10.1007/s00228-018-2558-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-2558-6