Abstract
Objectives
To develop a population pharmacokinetic (PK) model for vancomycin in Chinese neonates and infants less than 2 months of age (young infants) with a wide gestational age range, in order to determine the appropriate dosing regimen for this population.
Methods
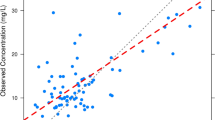
We performed a retrospective chart review of patients from the neonatal intensive care unit (NICU) at Children’s Hospital of Fudan University to identify neonates and young infants treated with vancomycin from May 2014 to May 2017. Vancomycin concentrations and covariates were utilized to develop a one-compartment model with first-order elimination. The predictive performance of the final model was assessed by both internal and external evaluation, and the relationship between trough concentration and AUC0–24 was investigated. Monte Carlo simulations were performed to design an initial dosing schedule targeting an AUC0–24 ≥ 400.
Results
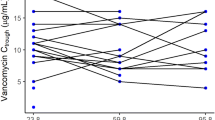
The analysis included a total of 330 concentration–time data points from 213 neonates and young infants with gestational age (GA) and body weight of 25–42 weeks and 0.88–5.1 kg, respectively. Body weight, postmenstrual age (PMA) and serum creatinine level were found to be important factors explaining the between-subject variability in vancomycin PK parameters for this population. Both internal and external evaluation supported the prediction of the final vancomycin PK model. The typical population parameter estimates of clearance and distribution volume for an infant weighing 2.73 kg with a PMA of 39.8 weeks and serum creatinine of 0.28 mg/dL were 0.103 L/h/kg and 0.58 L/kg, respectively. Although vancomycin serum trough concentrations were predictive of the AUC, considerable variability was observed in the achievement of an AUC0–24/MIC of ≥400. For MIC values of ≤0.5 mg/L, AUC0–24/MIC ≥400 was achieved for 95% of the newborn infants with vancomycin troughs of 5–10 mg/L. When the MIC increased to 1 mg/L, only 15% of the patients with troughs of 5–10 mg/L achieved AUC0–24/MIC ≥400. For MIC values of 2 mg/L, no infants achieved the target. Simulations predicted that a dose of at least 14 and 15 mg/kg every 12 h was required to attain the target AUC0–24 ≥ 400 in 90% of infants with a PMA of 30–32 and 32–34 weeks, respectively. This target was also achieved in 93% of simulated infants in the oldest PMA groups (36–38 and 38–40 weeks, respectively) when the dosing interval was extended to 8 h. For infants with a PMA ≥44 weeks, a dose increase to 18 mg/kg every 8 h was needed. The trough concentrations of 5–15 mg/L were highly predictive of an AUC0–24 of ≥400 when treating invasive MRSA infections with an MIC of ≤1 mg/L.
Conclusions
The PK parameters for vancomycin in Chinese infants younger than 2 months of age were estimated using the model developed herein. This model has been used to predict individualized dosing regimens in this vulnerable population in our hospital. A large external evaluation of our model will be conducted in future studies.





Similar content being viewed by others
References
Clark RH, Bloom BT, Spitzer AR, Gerstmann DR (2006) Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics 117(6):1979–1987
Jacqz-Aigrain E, Zhao W, Sharland M, van den Anker JN (2013) Use of antibacterial agents in the neonate: 50 years of experience with vancomycin administration. Semin Fetal Neonatal Med 18(1):28–34
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE (2003) Developmental pharmacology-drug disposition, action, and therapy in infants and children. N Engl J Med 349(12):1157–1167
Stockmann C, Roberts JK, Yu T, Constance JE, Knibbe CA, Spigarelli MG, Sherwin CM (2014) Vancomycin pharmacokinetic models: informing the clinical management of drug-resistant bacterial infections. Expert Rev Anti-Infect Ther 12(11):1371–1388
Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP (2009) Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66(1):82–98
Rybak MJ (2006) Pharmacodynamics: relation to antimicrobial resistance. Am J Med 119(6 Suppl 1):S37–S44
Rybak MJ (2006) The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis 42(Suppl 1):S35–S39
Craig WA (2003) Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin N Am 17(3):479–501
Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF, Infectious Diseases Society of America (2011) Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52(3):e18–e55
Camaione L, Elliott K, Mitchell-Van Steele A, Lomaestro B, Pai MP (2013) Vancomycin dosing in children and young adults: back to the drawing board. Pharmacotherapy 33(12):1278–1287
Gordon CL, Thompson C, Carapetis JR, Turnidge J, Kilburn C, Currie BJ (2012) Trough concentrations of vancomycin: adult therapeutic targets are not appropriate for children. Pediatr Infect Dis J 31(12):1269–1271
Frymoyer A, Guglielmo BJ, Hersh AL (2013) Desired vancomycin trough serum concentration for treating invasive methicillin-resistant staphylococcal infections. Pediatr Infect Dis J 32(10):1077–1079
Lanke S, Yu T, Rower JE, Balch AH, Korgenski EK, Sherwin CM (2017) AUC-guided vancomycin dosing in adolescent patients with suspected sepsis. J Clin Pharmacol 57(1):77–84
Frymoyer A, Hersh AL, El-Komy MH, Gaskari S, Su F, Drover DR, Van Meurs K (2014) Association between vancomycin trough concentration and area under the concentration-time curve in neonates. Antimicrob Agents Chemother 58(11):6454–6461
Le J, Bradley JS, Murray W, Romanowski GL, Tran TT, Nguyen N, Cho S, Natale S, Bui I, Tran TM, Capparelli EV (2013) Improved vancomycin dosing in children using area under the curve exposure. Pediatr Infect Dis J 32(4):e155–e163
Hahn A, Frenck RW Jr, Zou Y, Vinks AA (2015) Validation of a pediatric population pharmacokinetic model for vancomycin. Ther Drug Monit 37(3):413–416
Janssen EJ, Välitalo PA, Allegaert K, de Cock RF, Simons SH, Sherwin CM, Mouton JW, van den Anker JN, Knibbe CA (2015) Towards rational dosing algorithms for vancomycin in neonates and infants based on population pharmacokinetic modeling. Antimicrob Agents Chemother 60(2):1013–1021
Samardzic J, Allegaert K, Wilbaux M, Pfister M, van den Anker JN (2016) Quantitative clinical pharmacology practice for optimal use of antibiotics during the neonatal period. Expert Opin Drug Metab Toxicol 12(4):367–375
Sheiner LB, Rosenberg B, Melmon KL (1972) Modelling of individual pharmacokinetics for computer-aided drug dosage. Comput Biomed Res 5(5):411–459
Schwartz GJ, Feld LG, Langford DJ (1984) A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatr 104(6):849–854
Thomson Reuters clinical editorial staff (2011) Neofax 2011, 24th ed.
Marqués-Miñana MR, Saadeddin A, Peris JE (2010) Population pharmacokinetic analysis of vancomycin in neonates. A new proposal of initial dosage guideline. Br J Clin Pharmacol 70(5):713–720
Anderson BJ, Allegaert K, Van den Anker JN, Cossey V, Holford NH (2007) Vancomycin pharmacokinetics in preterm neonates and the prediction of adult clearance. Br J Clin Pharmacol 63(1):75–84
Ette EI, Williams PJ, Kim YH, Lane JR, Liu MJ, Capparelli EV (2003) Model appropriateness and population pharmacokinetic modeling. J Clin Pharmacol 43(6):610–623
Comets E, Brendel K, Mentré F (2008) Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Prog Biomed 90(2):154–166
van der Meer AF, Marcus MA, Touw DJ, Proost JH, Neef C (2011) Optimal sampling strategy development methodology using maximum a posteriori Bayesian estimation. Ther Drug Monit 33(2):133–146
Vinks AA (2011) Important role of population pharmacokinetic/pharmacodynamic modeling in pediatric therapeutics. J Pediatr 159(3):361–363
Anderson BJ, Holford NH (2011) Tips and traps analyzing pediatric PK data. Paediatr Anaesth 21(3):222–237
Vieux R, Hascoet JM, Merdariu D, Fresson J, Guillemin F (2010) Glomerular filtration rate reference values in very preterm infants. Pediatrics 125(5):e1186–e1192
Capparelli EV, Lane JR, Romanowski GL, McFeely EJ, Murray W, Sousa P, Kildoo C, Connor JD (2001) The influences of renal function and maturation on vancomycin elimination in newborns and infants. J Clin Pharmacol 41(9):927–934
Golper TA, Noonan HM, Elzinga L, Gilbert D, Brummett R, Anderson JL, Bennett WM (1988) Vancomycin pharmacokinetics, renal handling, and nonrenal clearances in normal human subjects. Clin Pharmacol Ther 43(5):565–570
Marsot A, Boulamery A, Bruguerolle B, Simon N (2012) Vancomycin: a review of population pharmacokinetic analyses. Clin Pharmacokinet 51(1):1–13
Ringenberg T, Robinson C, Meyers R, Degnan L, Shah P, Siu A, Sturgill M (2015) Achievement of therapeutic vancomycin trough serum concentrations with empiric dosing in neonatal intensive care unit patients. Pediatr Infect Dis J 34(7):742–747
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 635 kb)
Rights and permissions
About this article
Cite this article
Chen, Y., Wu, D., Dong, M. et al. Population pharmacokinetics of vancomycin and AUC-guided dosing in Chinese neonates and young infants. Eur J Clin Pharmacol 74, 921–930 (2018). https://doi.org/10.1007/s00228-018-2454-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-2454-0