Abstract
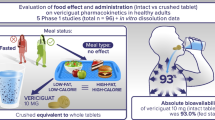
Objective. Objective of the study was the comparison of two nifedipine sustained-release products marketed in Europe. Maximum plasma concentration (Cmax) and area under the plasma–concentration curve (AUC) values were derived after administration of single doses (60 mg) of test product and reference product, both approved for once-a-day administration, to 24 healthy male volunteers either after an overnight fast or immediately after a high-fat American breakfast. The study was performed with a randomised, non-blinded, four-period crossover design. Within- and between-product comparisons were determined for fed versus fasted administration considering bioavailability and tolerability of all treatments. Furthermore, in vitro dissolution characteristics of both products were evaluated.
Methods. Plasma samples were assayed using a liquid chromatography-mass spectrometry method, and resulting pharmacokinetic parameters were determined model independently according to international requirements and the current European guidelines.
Results. Under fasted conditions the comparison of test and reference products showed a similar extent of bioavailability with a mean ratio of AUC(0– ∞ ) of 99% [95% confidence interval (CI) 86%, 114%], but significantly higher Cmax values resulting in a mean ratio of 169% (95% CI 139%, 206%). Accordingly, mean residence time and half-value duration values were smaller for the test product than the reference product. Under fed conditions, a pronounced food effect could be observed for the test product resulting in a pronounced increase of Cmax values. The affiliating point estimate was calculated as 340% with a 95% CI of 279%, 413%. However no remarkable influence of food intake was observed for the reference product.
Conclusion. Under fasting conditions the modified-release characteristics of the test product are less pronounced than the reference product. No relevant impact of food intake could be observed for the reference product when switching from fasted to fed state, whereas a significant loss of modified-release characteristics could be detected for the test product under fed conditions resulting in much higher maximum concentrations. Such a phenomenon has been described in literature as "dose-dumping effect".
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Accepted in revised form: 12 February 2002
Electronic Publication
Rights and permissions
About this article
Cite this article
Schug, B., Brendel, E., Wonnemann, M. et al. Dosage form-related food interaction observed in a marketed once-daily nifedipine formulation after a high-fat American breakfast. Eur J Clin Pharmacol 58, 119–125 (2002). https://doi.org/10.1007/s00228-002-0444-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-002-0444-7