Abstract
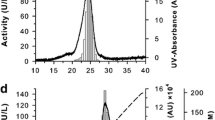
Phenoloxidase (PO) from the colonial ascidian Botryllus schlosseri was purified using two different chromatographic strategies. A three-step purification was developed in order to maintain enzyme activity, whereas an easier purification procedure was adopted to obtain enough PO for the production of specific polyclonal antibodies. The enzyme showed optimal pH and temperature values of 7.0 to 7.5 and 35 °C, respectively, and a K m value of 4.62 ± 0.76 mM was estimated using l-DOPA as substrate. A molecular weight of 160 kDa was determined after SDS-PAGE under non-reducing conditions. The addition of the reducing agent β-mercaptoethanol caused the disappearance of the 160 kDa band and the appearance of a new band at 80 kDa, suggesting that active PO is a dimer and the two subunits are linked by disulphide bridges.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 14 December 1998 / Accepted: 24 August 1999
Rights and permissions
About this article
Cite this article
Frizzo, A., Guidolin, L., Ballarin, L. et al. Purification and partial characterisation of phenoloxidase from the colonial ascidian Botryllus schlosseri . Marine Biology 135, 483–488 (1999). https://doi.org/10.1007/s002270050648
Issue Date:
DOI: https://doi.org/10.1007/s002270050648