Abstract
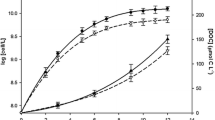
Cell growth of a coastal marine diatom, Chaetoceros sociale, in the presence of different premixed organic-Fe(III) complexes [EDTA-Fe(III) (100:1 and 2:1), citric-Fe(III) (100:1) and fulvic-Fe(III) (0.1, 0.2 and 1 ppm C)] and solid amorphous hydrous ferric oxide [am-Fe(III) or Fe(III) hydroxide] were experimentally measured in culture experiments at 10 °C under 3000 lux fluorescent light. Fulvic-Fe(III) (0.1 and 0.2 ppm C) and citric-Fe(III) (100:1) induced maximal cell yields of C. sociale. The order of cell yields was: fulvic-Fe(III) (0.1 and 0.2 ppm C) ≥ citric-Fe(III) (100:1) > EDTA-Fe(III) (2:1) ≫ solid am-Fe(III) > EDTA-Fe(III) (100:1) ≫ fulvic-Fe(III) (1 ppm C). The short-term iron uptake rates by C. sociale in fulvic-Fe(III) (0.1 and 0.2 ppm C) and citric-Fe(III) (100:1) media were about five to six times faster than those in EDTA-Fe(III) (100:1) and solid am-Fe(III) media. The dissociative precipitation rates of premixed organic-Fe(III) complexes in seawater at 10 °C were determined by simple filtration (0.025 μm) involving γ-activity measurements of 59Fe. The order of estimated initial Fe(III) dissociative precipitation rates of these organic-Fe(III) complexes in seawater were nearly consistent with those of cell yields in the culture experiments and short-term iron uptake rates by C. sociale [except for fulvic-Fe(III) (1 ppm C) medium]. In fulvic-Fe(III) (0.1 and 0.2 ppm C), citric-Fe(III) (100:1) and EDTA-Fe(III) (2:1) media, the concentrations of dissolved organic-Fe(III) complexes in initial culture experiments are prone to supersaturate under the culture conditions. The supersaturated dissolved organic-Fe(III) complex in seawater supplies biologically available inorganic Fe(III) species in culture media through its dissociation at high pH and high levels of seawater cations. Therefore, the natural dissolved organic-Fe(III) complexes supplied by riverine input may play an important role in supplying bioavailable iron in estuarine mixing system and coastal waters.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 6 September 1998 / Accepted: 8 April 1999
Rights and permissions
About this article
Cite this article
Kuma, K., Tanaka, J. & Matsunaga, K. Effect of natural and synthetic organic-Fe(III) complexes in an estuarine mixing model on iron uptake and growth of a coastal marine diatom, Chaetoceros sociale. Marine Biology 134, 761–769 (1999). https://doi.org/10.1007/s002270050593
Issue Date:
DOI: https://doi.org/10.1007/s002270050593