Abstract
The presence of a seasonally variable biogenic habitat (eelgrass, Zostera marina) increased the spring–summer variability of associated nekton relative to unvegetated bare tidal flat. This spatio-temporal pattern emerged because most eelgrass-associated taxa tracked the decline in eelgrass biomass from summer to spring, but in one case reached greater density in spring when predation intensity was low. Among 21 taxa (26,884 individuals) captured, a strong correlation arose between structure association and summer dominance, and certain functional traits, in particular morphology and on- vs. off-bottom position, were strong predictors of eelgrass association. Structure-associated taxa were slender-bodied and pelagic schooling fishes, while habitat generalists or bare-associated taxa were more consistent seasonally, primarily benthic, and cryptically colored with sand. Estuarine use (transient, reproducing, or estuarine resident) was not a strong predictor of structure association or seasonality. Because an identical sampling design was used in five regions of Washington State, USA, coarse-scale (> 100 km) differentiation in nekton assemblages was identified, representing less of the total variation than across seasons but more than across different habitats. While regional nekton differences were attributable in part to geographic distance and eelgrass morphotypes, the most seasonally variable nekton were at sites with morphotypes adding the least vertical structure but highest density. These results support two mechanisms that increase seasonal variability of taxa using structured habitats, including both bottom-up provision of habitat and resources, and subsequent possibilities for negative interspecific interactions and top-down control.
Similar content being viewed by others
Introduction
The trophic structure of coastal grass-bed communities changes seasonally (Livingston 1984), in some cases both more dramatically and more consistently than in other shallow-water habitats (Cote et al. 2013). When populations show seasonal variation in abundance, this may be attributed to the timing of reproductive events or movement among habitats (Ribeiro et al. 2012). However, habitat structure, resources, and predation risk can also change seasonally (Hines et al. 1990; Hovel et al. 2002), providing explanations for seasonal population change that depend on species interactions, rather than autecological phenology. Attributing mechanisms for seasonal changes in community structure, especially for higher trophic level species, is accordingly challenging.
Seasonal use of estuaries provides a functional trait for categorizing nekton (Potter et al. 2015), but use of eelgrass as habitat may depend on other traits such as long, narrow body plans or vertical distribution above the sediment (Hori et al. 2009, Gross et al. 2017, 2018). Additionally, whether organisms receive protection or more intense predation pressure in seagrass can be influenced by body size, trophic position, and feeding mode (Horinouchi 2007). Seasonal patterns in seagrasses are well established (Unsworth et al. 2012; Clausen et al. 2014), but—perhaps due to multiple pathways by which nekton respond to structure and/or seasonality—seasonal use of seagrass habitat by nekton has had more variable outcomes. In some cases, nekton biomass sampled in seagrass tracks the seasonality of this biogenic habitat (Adams 1976; Livingston 1984; Able et al. 2002; Tuya et al. 2006; Mateo-Ramirez and Garcia-Raso 2012). However, other cases appear more nuanced. In seagrass within tidal creeks of the Rio Formosa Lagoon, Portugal, total abundance of fishes did not change seasonally because some groups were more abundant in autumn and winter, and others in spring and summer (Ribeiro et al. 2012). Similarly, in a seagrass bed in China, many mobile taxa were trapped in greater numbers when seagrass size and density increased, but not all (Xu et al. 2016). On the US Gulf Coast, nekton abundances were consistent from summer to fall in continuous seagrass, but decreased from summer to fall in small patches as shrimp were particularly dense in summer in this landscape type (Hensgen et al. 2014).
In addition to seasonality, nekton assemblages vary spatially among different habitat types in close proximity, as well as among similar habitats at geographic distances. Whitfield (2017) suggests that ca. 100 km is typically far enough apart that fish assemblages in the same seagrass differ. At smaller scales, bare areas > 100 m from structure can have fewer fish than those within 10 m of structure (Ferrell and Bell 1991), and large seagrass areas may have lower density fish than small seagrass areas because the latter have extensive edges suitable for schooling species (Horinouchi 2007). Fish assemblages frequently differ by seagrass species (Rotherham and West 2002, Hyndes et al. 2003), and are usually different in comparisons of vegetated and unvegetated habitat (Whitfield 2017). Body size and coloration represent critical functional traits as size determines whether seagrass provides space for hiding or an obstruction (Hyndes et al. 2003), and color can provide crypsis with sand, mud, or vegetation. Benthic species may prefer less structural complexity than pelagic species (Hori et al. 2009).
Given spatial and temporal differences in nekton, these two axes may also interact. For instance, summer to fall change in nekton assemblages was more pronounced in seagrass than in unvegetated habitats in eastern Canada (Cote et al. 2013). At an intertidal site in the Wadden Sea, juveniles of seagrass-associated fish generally increased from June to September with eelgrass biomass, whereas those using bare habitat had more consistent monthly catches (Polte and Asmus 2006). In Japan where both annual and perennial life histories of eelgrass occur, positive correlation of fish abundance and eelgrass was apparent for annual eelgrass, which was primarily present from April to June, whereas fish in the guild of permanent residents were present year-round in perennial eelgrass (Sato et al. 2016). While we summarize these published studies in terms of habitat-specific seasonality, another logical framing is that habitat structure matters more for nekton assemblages during seasonally high biomass of seagrass—that is, season-specific habitat effects. Seasonality in the degree of habitat provision by seagrass has also been reported for infaunal assemblages (Włodarska-Kowalczuk et al. 2014).
The variation in assemblages of nekton expressed in space and time may be reciprocally related to trophic interactions. Fish may accumulate in seagrass due to protection from predators (Heck and Orth 1980), and seasonal increases of fish or crab populations can cause top-down effects on prey (Livingston 1984, Hines et al. 1990). Seasonal migrations into seagrass habitat may depress populations of resident species due to predation on small life stages or resource competition (Kneib and Knowlton 1993). Yet predation in seagrass shows a wide range of responses to increased shoot density, likely reflecting the range of adaptations of prey for avoiding predators and of predators for attacking prey (Horinouchi 2007). Many feeding functional groups are represented among mobile taxa captured by a common net size (Elliott et al. 2007). Piscivores and large zoobenthivores that include crabs and shrimp in their diet may actually reduce densities of other nekton through intraguild predation, whereas other zoobenthivores may indirectly enhance epiphyte loads by removing mesograzers from seagrass leaves (Duffy et al. 2005).
Our hypotheses regarding nekton assemblages across five regions of Washington State are:
-
1.
Habitat will determine the amount of seasonal variability in nekton. Interactions of season and habitat effects could emerge for two reasons, which are distinct but not mutually exclusive: seasonality is expected to be more pronounced in seagrass than bare habitat, and habitat distinctions are expected to be reduced for nekton in spring, when seagrass is sparse, relative to the peak summer growth season for seagrass.
-
2.
Sites in different regions will have distinct nekton assemblages, but more so as the distance between sites or seagrass ecotypes increases. This hypothesis could extend not just to the composition of nekton, but also to its seasonal or habitat variation.
-
3.
Taxa that respond primarily to habitat or resource availability are expected to increase in abundance in seagrass from spring to summer. However, we anticipate decreases during this seasonal change for taxa most susceptible to intraguild predation. To distinguish among potential mechanisms underlying habitat-specific seasonality, we categorized nekton taxa by several functional traits and tested for habitat-specific seasonality in predation intensity.
These hypotheses suggest linked structural and functional changes in nekton assemblages, in which seasonal responses in some species modify those of others via species interactions, but overall most intense trophic interactions occur during summer.
Methods
Study sites
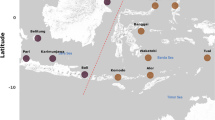
Surveys of nekton and predation intensity were carried out at five sites located in distinct oceanographic regions of Washington State, USA. Sites were selected to include a mosaic of low-intertidal soft-sediment habitats consisting of native eelgrass (Zostera marina) interspersed with unvegetated tidal flat. Sites were located in North Puget Sound (NPS), South Puget Sound (SPS), North Hood Canal (NHC), South Hood Canal (SHC), and the coastal estuary (CE) of Willapa Bay (Fig. 1a). These five regions have bathymetric and oceanographic features that make their water properties distinctive in terms of residence time, temperature, and chemistry (Babson et al. 2006; Banas et al. 2007, 2015). The sites range in geographic distance from 76 to 547 km by water (Supplemental Material Table S1). Summer-season nekton communities were documented in 2015 at three of these sites, showing large regional differences that exceeded differences between vegetated and unvegetated habitats (Gross et al. 2017). Eelgrass at the five sites spanned a range of morphotypes (i.e., nearly order-of-magnitude difference in canopy height), but due to inverse relationships between shoot size and shoot density (Fig. 1b), leaf area index and above-ground biomass were similar (Gross et al. 2017). In Washington State, spring-season eelgrass biomass is typically depressed by half relative to summer (Thom 1990; Ruesink et al. 2010; Ruesink and Rowell 2012; Valdez et al. 2017).
a Locations of five study sites in Washington State, USA, where nekton and predation intensity were concurrently sampled across low-intertidal habitats. b Eelgrass (Zostera marina) showed an inverse relationship between size and density across sites. Error bars show SE of four subsites per site, where each subsite value was based on ten quadrats
Sampling design
At each site, sampling was carried out during daylight in summer 2016 (July–August) and spring 2017 (March–May), with each site being sampled over 2–5 days during spring tide periods when water levels fell low enough to use a custom beach seine. The seine was used when the water dropped to between 0.2 and 0.8 m in depth; however, based on earlier work with underwater video, nekton assemblages remain similar through the tidal cycle (Gross et al. 2018). The seine was 1 m tall with 3-mm mesh. Its wings of 6 m length were pulled out by two people into a circle of ca. 11 m2 area. Subsequently, the wings were pressed together, chasing the captured organisms into the cod end. The cod end was held in water as individual organisms were counted, identified, and immediately released.
For summer 2016 sampling, we found areas near mean lower low water in which eelgrass was patchy, thereby interspersed with bare. Selected eelgrass patches were ca. 10,000 m2, except for two smaller patches at SPS (85 and 909 m2) and one at CE (1255 m2); also, a larger meadow was sampled at three of the sites. Four subsites were chosen at each of the five sites, and at each subsite, two seines were conducted within eelgrass, two on unstructured (bare) tidal flat, and two that encircled the border of these two habitats, such that half the area of the seine contained eelgrass. These habitat types consequently provide a gradient of structural complexity (Fig. 1b), with edge as an intermediate, rather than exceptional, habitat (Gross et al. 2018). Interior eelgrass and bare habitats were typically sampled at 3 m from the edge and not more than 50 m from the edge. Sampling in the summer was repeated with identical methods the following spring. Given five sites, four subsites per site, three habitats (patches) per subsite, seined twice in summer and twice in spring, a total of 240 seines was conducted.
Predation intensity
Predation intensity was determined by loss of baits (1 cm2 dried squid mantle) over 24 h (Duffy et al. 2015). These baits were attached to thin 10-cm-long monofilament line, tethered to a bamboo stake. Knots at both ends of the line were reinforced with a drop of cyanoacrylate glue. Twenty baited stakes were inserted 1 m apart in each of the three habitats for a 24-h period. Those placed at the edge habitat type followed the line where eelgrass was present on one side and absent on the other. Baits just touched the sediment, making them accessible to both benthic and pelagic predators. Baits were only scored as a predation event if the entire bait (but not the tether) was missing after 24 h. Deployment occurred during the same low-tide period as, and in close proximity to where, seines were carried out. Baits were placed in each of the 60 habitat patches in summer 2016, but in a subset of 23 in spring 2017 due to shorter periods of access to the sites at low tide.
Data analysis
We used non-metric multidimensional scaling (NMDS) to visualize differences in nekton assemblages and permutational analysis of variance (PERMANOVA) for statistical analysis. Rare taxa were removed from the analysis (see Results), and remaining counts of 21 taxa were log(x + 1)-transformed to downweight the most common taxa. Both NMDS and PERMANOVA were based on Bray–Curtis similarities. Fixed factors in this analysis were season (two levels), site (five levels), habitat (three levels), and all two- and three-way interactions. Subsite was entered as the strata argument of the adonis function (vegan package in R; Oksanen et al. 2015, R core team 2015), which accounts for the nested design of sampling all habitats at each subsite. 9999 randomizations were carried out and significance assessed at α = 0.05. Given that hypothesis (1) predicted a season × habitat interaction, we followed up with PERMANOVA applied to each habitat separately, to determine how much seasonal difference occurred in each habitat. A complementary analysis testing how much habitat variation occurred in each season is presented in supplemental material (Table S2).
In accordance with hypothesis (2), we expected that site-level differences could be due to geographic proximity or eelgrass morphotype. We tested for spatial correlation in our multivariate nekton data through application of a Mantel correlogram. This used the dissimilarity matrix for nekton summed across all seines per site (Bray–Curtis on log(x + 1)-transformed counts) and a dissimilarity matrix of distances (by water) among sites. Note that we were able to sum counts across seines to examine site-level differences because the sampling effort was identical at each site. We separated summer and spring data in case geographic proximity mattered differentially across seasons. The test for spatial correlation employed the mantel.correlog function in package vegan (Oksanen et al. 2015). To test for eelgrass morphology, each of the 20 subsites in the study was described by the canopy height of interior eelgrass. We replaced the site factor in three-factor PERMANOVA with the continuous variable of canopy height, using site instead as the object of the strata command, as a random effect.
Hypothesis (3) proposed that species-specific patterns of spatio-temporal variation could be due to bottom-up or top-down control and could cluster according to functional traits. Accordingly, we calculated metrics of seasonality, habitat and edge association for each taxon captured in seines. The design of our study presented up to 60 paired summer–spring samples and 40 paired samples of different habitats per subsite. For each of these paired samples in which a taxon was present at least once, we calculated metrics of seasonality and habitat association that essentially represented the log-ratio of counts under two conditions and could then be summarized by mean and standard error. For xsu and xsp as the count of a taxon in the two seines per patch in summer and spring, seasonality was calculated as ln((xsu + 1)/(xsp + 1)), giving values > 0 for taxa occurring at higher abundance in summer. For xie and xb as the count of a taxon in the two seines per patch in interior eelgrass and bare, respectively, at a subsite, habitat association was calculated as ln((xie + 1)/(xb + 1)), giving values > 0 when disproportionately in eelgrass. Finally, an index of edge association followed the logic that seines at the edge were half-bare and half-eelgrass, so should have an intermediate count of each taxon. For xe as the count in two seines at the edge at a subsite, edge association was calculated as ln((xe + 1)/((xie + xb)/2 + 1)). We grouped the 21 major nekton taxa into three categories of estuarine use (Table 1): resident taxa; those found in estuaries specifically during the reproductive or settlement phase of their life cycle; and transient taxa straggling or moving into estuaries for purposes other than reproduction. Anadromous salmon were included in this last category. These taxa were also grouped by vertical distribution in the water column (benthic on-bottom vs. pelagic or epifaunal off-bottom; Table 1). Then we tested whether seasonality, habitat, or edge association differed by traits of estuarine use or vertical distribution based on linear models, weighted by the sample size per taxon, since some taxa were found in more patches than others. We also tested for correlations between habitat association and seasonality across taxa (Pearson’s r).
To analyze predation intensity, as the fraction of baits lost over 24 h, we structured a three-factor analysis in the same manner as for nekton community analyses, with baits removed (relative to those still present) as the response variable, fixed effects of season, site, habitat, and their two- and three-way interactions, and a random effect of subsite. We ran this analysis with all available data and a second time including only those seven subsites (21 patches) that were fully sampled in both seasons. These three-factor mixed effects models, assuming binomial error structure, used the glmer function in the lme4 package in R (Bates et al. 2015). We selected the best model based on a comparison of Akaike’s Information Criterion (small sample size-adjusted, AICc), which penalizes model complexity (Burnham and Anderson 2002).
Results
Hypothesis (1): community variation by season and habitat
In total, 27,016 individual organisms were captured in seines during summer 2016 (16,679, 62%) and spring 2017 (10,337, 38%). For multivariate analysis, we excluded rare taxa found at low total numbers (< 20), unless they were frequent enough to occur in ten or more seines. This removed 18 taxa and some additional poorly identified individuals, i.e., “hermit crab” or “sculpin larva”. Twenty-one taxa (26,884 individuals) remained in the analysis (Table 1). At the whole-assemblage level, most variation was a function of season and site, but assemblages also differed among habitats (Table 2, Fig. 2). All two-way interactions were significant. For each habitat separately, seasonal differences increased with habitat complexity; that is, the main effect of season explained more nekton variation in interior eelgrass (r2 = 0.38) than edge (r2 = 0.30) or bare (r2 = 0.16; Table 2). On the other hand, these habitats showed similar nekton variation at the spatial scale of sites (r2 = 0.19–0.24). The complementary analysis to understand this season × habitat interaction was a two-factor PERMANOVA separately for summer and spring data: the habitat factor had a larger r2 value for summer (0.11) than spring (0.04, Supplemental Material Table S2). On a site-specific basis, nekton also demonstrated greatest habitat differences in summer, when 13 of 15 pairwise habitat comparisons showed statistical differences, as compared to 4 of 15 in spring (Supplemental Material Table S3). Based on r2 values, bare and eelgrass (interior) assemblages were more distinct from each other than either endpoint in comparison to edge, showing edge assemblages to be intermediate (Supplemental Material Table S3).
Hypothesis (2): spatial variation in nekton in accordance with proximity or eelgrass morphotype
Hypothesis (2) invoked geographic proximity or eelgrass morphotype as factors that could generate site effects and interactions with site, as in results just reported for three-way PERMANOVA. Geographic proximity and morphotype dissimilarity were variables suited to different types of analyses, but were not redundant predictors (Pearson’s r = 0.06, N = 10). In terms of geographic proximity, significant spatial correlation in nekton assemblages occurred only in summer and at 123 km but not greater (Mantel correlation = 0.56, P = 0.006); no correlation was evident at this scale for spring data (Mantel correlation = − 0.24, P = 0.28). When subsite-specific canopy height was included as a predictor in place of site in PERMANOVA, nekton assemblages differed significantly with canopy height and its interaction with season (Table 2). This interaction is consistent with the amount of seasonal variation at each site, which constituted more of the total nekton variation at sites with shorter eelgrass canopy. In order of small to large eelgrass morphotype, r2 values for season were 0.48, 0.41, 0.34, 0.46, and 0.34 (Supplemental Material Table S4).
Hypothesis (3): seasonality and habitat association by taxon
Most of the 21 nekton taxa were more abundant in seines in summer than spring, while only two showed the reverse seasonal abundance (Fig. 3a). These were juvenile salmonids, which were expected to be outmigrating in spring, and grass shrimp (Hippolytidae), which were the smallest taxon caught in seines (Table 1). Seasonality was indistinguishable across the three general categories of estuarine use (Resident, Reproductive, Transient, F2,18 = 0.36, P = 0.7) and also did not differ with on-bottom (benthic) or off-bottom vertical distribution (F1,19 = 0.01, P = 0.9). Nekton taxa were about evenly divided among those showing eelgrass association and no habitat distinction, with just two (English sole, surf smelt) being significantly bare associated (Fig. 3a). As with seasonality, habitat association did not differ with estuarine use (F2,18 = 0.98, P = 0.4), but, in contrast, benthic taxa showed lower eelgrass association than those with off-bottom vertical distribution (F1,19 = 9.3, P = 0.006). Surf smelt and juvenile salmonids appear to violate the eelgrass association of off-bottom taxa (positioned on the left-hand side of Fig. 3) but had small weights in the analysis (Supplemental Material Table S5), as well as cautioning whether morphology and vertical position serve as habitat predictors for transient taxa in estuaries. Across taxa, those with stronger eelgrass association were more likely to have higher summer than spring densities (Pearson’s r = 0.53, N = 21, P = 0.01). Hippolytid shrimp departed strongly from this correlation, as seen by their placement in the lower right-hand quadrant of Fig. 3a. Positive edge association was not detected in any taxon (Fig. 3b), while several taxa were calculated to avoid edge, with significantly more avoidance by off-bottom than benthic taxa (F1,19 = 6.4, P = 0.02), and no difference by estuarine use (F2,18 = 0.15, P = 0.9). However, no overall negative correlation existed between edge and eelgrass association (Pearson’s r = − 0.38, N = 21, P = 0.09). For no taxa were edges the least-used habitat; even those with edge avoidance still used edges as much as bare (Table 1).
Spatio-temporal patterns of 21 nekton taxa captured in seines on low-intertidal flats of Washington State, USA. Horizontal axis shows the log ratio of abundances in eelgrass relative to bare. Vertical axes depict, a log ratio of abundances in summer 2016 relative to spring 2017, and b log ratio of abundances in seines at the edge of eelgrass relative to the average of eelgrass and bare. Error bars are standard errors; sample size is shown in Supplemental Table S5. Symbols designate functional groups of estuarine use and vertical position. a 1 = shiner perch; 2 = stickleback; 3 = snake prickleback; 4 = midshipman; 5 = dungeness crab; 6 = graceful crab; 7 = starry flounder; 8 = bay shrimp; 9 = English sole. b 1 = surf smelt; 2 = salmonid juveniles; 3 = staghorn sculpin; 4 = red rock crab; 5 = padded sculpin; 6 = dock shrimp; a = arrow goby; b = kelp crab; c = saddleback gunnel; d = pipefish; e = crescent gunnel; f = grass shrimp
Hypothesis (3): predation intensity
Fewer baits were removed in a day during spring (32%, 142/441) than summer season (70%, 810/1156). The magnitude of this seasonal difference, but not the direction, differed across sites (site × season interaction, Supplemental Material Table S6, Fig. 4). Overall, bait removal was similar in bare (60%, 308/516), edge (58%, 305/522), and eelgrass habitat (63%, 330/520). The top model for all bait-removal data included season, site, and their two-way interaction but no habitat difference (df = 10, AICc = 450.9), whereas for the subsites sampled in both spring and summer, this model (df = 7, AICc = 230.2) was essentially equivalent to one also including a habitat × site interaction (df = 13, AICc = 229.7; Supplemental Material Table S6). However, mean values for bait removal at each site never differed by more than 14% in the three habitats.
Discussion
Scales of spatio-temporal variability in nekton
Structural complexity from biogenic species such as seagrass often increases the diversity and abundance of associated mobile taxa (Heck et al. 1989; Hughes et al. 2002; Ferraro and Cole 2010). Incorporating seagrass density, canopy height, patch size, or distance to edge in study design has revealed scales of spatial variation (Bell and Westoby 1986a; Boström et al. 2006). What we address here is whether this modification changes seasonally, and if so, can we infer why? We expected a spatio-temporal (season × habitat) dynamic for nekton simply because the habitats themselves become more distinct as eelgrass increases its canopy height and above-ground biomass in late summer in Washington State (Thom 1990; Ruesink et al. 2010; Ruesink and Rowell 2012; Valdez et al. 2017). Consequently, summer seagrass provides the greatest contrast with nearby unvegetated areas, which may cause seasonal changes of nekton in seagrass to be more consistent than in unvegetated habitats (Cote et al. 2013). When seagrass biomass is seasonally lower, distinctions between nekton using seagrass and bare habitats tend to be muted (Able et al. 2002; Xu et al. 2016). Our results supported a general pattern of higher seasonal variability for nekton in eelgrass than bare habitat (Fig. 2, Table 2). Additionally, nekton assemblages were more distinct across habitats in summer than in spring.
Edges may constitute an intermediate habitat type in cases where edge effects are weak (Vonk et al. 2010), but an exceptional habitat if organisms are attracted to edges or interact strongly there (Smith et al. 2008, 2011). Based on past work in Washington State estuaries (Gross et al. 2018), we anticipated that nekton assemblages at edges would be intermediate between eelgrass and bare. Thus, we made no predictions in hypothesis (1) that specifically referred to edges but rather included them in tests across a gradient of structural complexity. On a taxon-specific basis, none reached highest density at edges. However, we documented a pattern that off-bottom species, which were generally strongly eelgrass associated, occurred at lower abundance at edges than would be expected from these seines surrounding half eelgrass and half bare areas (Fig. 3b). Edges were not avoided relative to bare—that is, abundances were typically intermediate (Table 1)—but the eelgrass there was used less by off-bottom taxa than interior eelgrass just a few meters farther into a patch. Compared to edge, interior eelgrass also encourages slower movement and more feeding by some off-bottom taxa (Gross et al. 2018).
In addition to habitat responses, nekton in seagrass diverge at coarser (ca. 100 km) spatial scales, which has been attributed to local recruitment dynamics (Rotherham and West 2002) but could also reflect distinct estuarine conditions or structural complexity (Hori et al. 2009). We confirmed differences in multivariate community structure at this 100-km scale, while also identifying spatial correlation in summer—that is, greater distances among the five sites generated more distinct communities, including some taxa that were not observed at all sites (Table 1). We were also able to substitute canopy height as a predictor and explain a portion of the site-level variation in nekton and their seasonal variability (Table 2). Why might sites with shorter canopies have more variable nekton? First, other sites with taller canopies may not vary in a range that matters to nekton. Second, seasonal variation occurs in shoot density rather than canopy height at short-morphotype sites (Ruesink and Rowell 2012), which may matter more than canopy height. Finally, nekton may consist of more seasonally variable taxa at short-canopy vs. tall-canopy sites, for instance, 84% of all individuals caught at the most seasonally variable site (SPS) were summer-dominant shiner perch or spring-dominant grass shrimp, whereas these taxa constituted no more than 65% at other sites. Seagrass can vary independently in biomass, height, density and structural complexity, and these factors can differ as drivers by location or taxon (Hovel et al. 2002; Hori et al. 2009). It is also worth keeping in mind that nekton may distribute according to seagrass morphotypes among sites in a different manner than within sites (Bell and Westoby 1986a, b).
Seasonality in the across-habitat distribution of nekton has many possible underlying reasons, which range from autecological reproductive timing or migration (Ribeiro et al. 2012) to synecological responses to resource availability or top-down control (Horinouchi 2007). Some aspects of our observational study fortuitously allowed us to distinguish potential mechanisms that might otherwise have been confounded. First, at the regional scale, eelgrass morphotype was not spatially correlated, so we could separately distinguish contributions of canopy height and geographic proximity to site-level variation in nekton. A drawback was that these predictors were not included in a common analysis to determine how much of total site-level variation they could explain. Second, predation intensity gave quite distinct predictions about nekton distributions relative to the bottom-up driver of eelgrass amount. Due to the homogeneity of predation intensity across habitats (Fig. 4), resources related to structural complexity provided a more logical explanation for habitat differences in community structure. Also, top-down effects potentially reducing nekton abundance were more prevalent in summer than spring (Fig. 4), at the same time as bottom-up effects would be expected to increase nekton abundances, this latter being the case for many taxa in our study. A drawback was that we measured predation intensity over short periods and with baits that may not mimic live predator–prey interactions.
Rather than eelgrass reducing predation, our results match about a quarter of the studies reviewed by Horinouchi (2007) in which predation was unaffected or increased in seagrass. In tropical deployments, squid baits were more rapidly removed in structured habitats of reef and seagrass than in unvegetated sand (Duffy et al. 2015). However, as predation generally increases in seagrass at lower latitudes (Reynolds et al. 2018), our results stimulate the question of whether the seagrass vs. bare comparison of predation risks could vary latitudinally. Because these baits have no predator-avoidance behavior, their removal is likely to be a function of predator density, rather than any habitat-mediated encounter rate (per capita effect of predators). However, living organisms may find more predator protection in eelgrass through escape responses using structure to hide. We suspect that baits were eaten by cancrid crabs and sculpins, based on their diets (Stevens et al. 1982; McPeek et al. 2015; Duffy et al. 2015), generalist habitat use coinciding with homogeneous bait removal across habitats (Figs. 3, 4), and images captured on remote video (pers. obs., B. Dumbauld, pers. comm.). In any case, we have more evidence that predation intensity is linked reciprocally to seasonal variation in nekton assemblages than to across-habitat distributions of nekton.
Spatio-temporal variability in relation to functional types
Substantial insight into drivers of community structure can emerge from a functional lens (Elliot et al. 2007). The striking correlation that we observed between seasonality and habitat association across taxa offers a logical proximate explanation for spatio-temporal variability in nekton assemblages (Figs. 2, 3). Each estuarine use functional type had representatives spanning the continuum of observed seasonality (Fig. 3). In some locations, seagrass is used as nursery habitat by taxa occupying estuaries for a portion of their life cycle (Heck et al. 2003; McDevitt-Irwin et al. 2016), whereas other locations have estuarine residents as seagrass-associated taxa (Heck et al. 1989; Franco et al. 2006). So, while estuarine use functional type has been helpful to explain habitat associations in particular cases, it is worth keeping in mind that the functional traits of taxa using seagrass differ widely geographically (Whitfield 2017). In contrast, in Washington State estuaries, vertical position helped distinguish taxa across a continuum of habitat association. Eelgrass-associated taxa have two body shapes that suit them to eelgrass use: shiner perch and sticklebacks swim well off the bottom among eelgrass leaves, and pipefish (and gunnels, despite benthic position) have elongate body forms that are cryptic in eelgrass and/or allow movement through small spaces. Those that also peak in summer tend to feed on invertebrates that densely populate eelgrass leaves (Barry et al. 1996; Hughes 1985; Ryer and Orth 1987; Spilseth and Simensted 2011), thus supporting a bottom-up response to resources or habitat availability. The exception, hippolytid (grass) shrimp, was the taxon most likely to be influenced by top-down effects in summer due to their small size and susceptibility to intraguild predation. Predation can strongly influence the diversity and abundance of crustacean communities using seagrass (Boada et al. 2018).
Many of the taxa at the other end of the continuum are benthic with coloration mimicking sand and mud, which is often the case for taxa that show no augmentation in seagrass (Horinouchi 2007). These include bay shrimp, gobies, flatfish, crabs, and sculpins. The lack of habitat association for crabs in our study needs to be placed in context of substantial past work focused on Dungeness crab habitat. This taxon has a long pelagic larval period, and new recruits appear disproportionately in structured habitats (Fernandez et al. 1993). However, by the time the crabs have grown for a year, structure becomes an impediment to long-distance movement onto tidal flats for foraging (Holsman et al. 2006). The crabs in our seines were mostly in this latter ontogenetic stage, yet showed no avoidance of eelgrass habitat, possibly because most of our sampling was in small eelgrass patches. While generalist habitat use and reduced seasonality appeared to align (Fig. 3), the seasonal span of sampling means that this association should be viewed cautiously. Indeed, our spring–summer sampling was insufficient to reveal seasonal changes in flatfish, which probably recruit in late winter given their average total length of 3 cm in spring but 6.5 cm in summer (Gross et al. 2019) and in bay shrimp, which typically peak in winter (Hosack et al. 2006).
Juvenile salmonids constitute a group of several species of anadromous fishes, sampled as juveniles as they migrate through estuaries from fresh to saltwater. Due to commercial importance and protected status of many populations, strong interest exists in any nursery habitat role of eelgrass for juvenile salmonids (Plummer et al. 2013). Their body plan and vertical position would tend to suggest eelgrass association. However, even after combining salmonid species, the capture data provide a weak test of habitat association due to the extremely patchy distribution of these fish. 573 pink salmon (Oncorhynchus gorbuscha) were captured in one seine in bare habitat at NHC, and 113 chum salmon (Oncorhynchus keta) were captured in one seine in bare habitat at CE, collectively constituting 94% of all juvenile salmonids recorded. Statistically, the null hypothesis that juvenile salmonids appear equally across vegetated and unvegetated low intertidal habitats could not be rejected. For some salmonid populations, feeding or protection in eelgrass may be advantageous (Semmens 2008; Kennedy et al. 2018), and for others little habitat association is evident (Dumbauld et al. 2015). In our results, juvenile salmonids moving through estuaries in spring did so prior to the summer rise in predation intensity, which gives their seasonality a potential adaptive benefit.
Much of what we report from this two-season comparison of nekton recapitulates the model developed by Livingston (1984) following temporally intensive sampling of grass beds in the Gulf of Mexico, in which fishes generally increase with the growth of vegetation, causing small invertebrates to decline in summer. To this seasonal comparison, we added two spatial dimensions: distinct habitats within sites and similar habitats across oceanographic basins. In this regard, our study design was similar to that of Franco et al. (2006), as well as methodologically in the use of small beach seines that effectively sample both structured and unstructured habitats. As in the case of Venice Lagoon (Franco et al. 2006), despite coarse-scale geographic variation in nekton, habitat-specificity emerged for many taxa. Within-site replication in our study, plus sites distributed geographically at distances typically resulting in distinct nekton assemblages (Whitfield 2017), undoubtedly contributed to the relatively strong site effects: second behind season and generally more important than habitat for community structure. Our study provides novel insight into the seasonal role of eelgrass as structured habitat, particularly given morphotype as a predictor of multivariate response, and identifies nekton functional types that respond seasonally through either bottom-up or top-down mechanisms. An on-going challenge exists to build a robust link between the functional representation in communities and seasonal dynamics within a habitat mosaic.
References
Able KW, Fahay MP, Heck KL, Roman CT, Lazzari MA, Kaiser SC (2002) Seasonal distribution and abundance of fishes and decapod crustaceans in a Cape Cod estuary. Northeast Nat 9:285–302
Adams SM (1976) The ecology of eelgrass, Zostera marina (L.), fish communities. 1. Structural analysis. J Exp Mar Biol Ecol 22:269–291
Babson AL, Kawase M, MacCready P (2006) Seasonal and interannual variability in the circulation of Puget Sound, Washington: a box model study. Atmos Ocean 44:29–45
Banas NS, Hickey BM, Newton JA, Ruesink JL (2007) Tidal exchange, bivalve grazing, and patterns of primary production in Willapa Bay, Washington, USA. Mar Ecol Prog Ser 341:123–139
Banas N, Conway-Cranos L, Sutherland D, MacCready P, Kiffney P, Plummer M (2015) Patterns of river influence and connectivity among subbasins of Puget Sound, with application to bacterial and nutrient loading. Estuaries Coasts 38:735–753
Barry JP, Yoklavich MM, Cailliet GM, Ambrose DA, Antrim BS (1996) Trophic ecology of the dominant fishes in Elkhorn Slough, California, 1974–1980. Estuaries 19:115–138
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1-48. https://doi.org/10.18637/jss.v067.i01
Bell JD, Westoby M (1986a) Importance of local changes in leaf height and density to fish and decapods associated with seagrass. J Exp Mar Biol Ecol 104:249–274
Bell JD, Westoby M (1986b) Variation in seagrass height and density over a wide spatial scale: effects on common fish and decapods. J Exp Mar Biol Ecol 104:275–295
Boada J, Pagès JF, Gera A, Macpherson E, Santana Y, Romero J, Alcoverro T (2018) The richness of small pockets: decapod species peak in small seagrass patches where fish predators are absent. Mar Environ Res 142:1–6
Boström C, Jackson EL, Simenstad CA (2006) Seagrass landscapes and their effects on associated fauna: a review. Estuar Coast Shelf Sci 68:383–403
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, Berlin
Clausen KK, Krause-Jensen D, Olesen B, Marbà N (2014) Seasonality of eelgrass biomass across gradients in temperature and latitude. Mar Ecol Prog Ser 506:71–85
Core Team R (2015) R: A Language and Environment for Statistical Computing. R foundation for statistical computing, Vienna
Cote D, Gregory RS, Morris CJ, Newton BH, Schneider DC (2013) Elevated habitat quality reduces variance in fish community composition. J Exp Mar Biol Ecol 440:22–28
Duffy JE, Richardson JP, France KE (2005) Ecosystem consequences of diversity depend on food chain length in estuarine vegetation. Ecol Lett 8:301–309
Duffy JE, Ziegler SL, Campbell JE, Bippus PM, Lefcheck JS (2015) Squidpops: a simple tool to crowdsource a global map of marine predation intensity. PLoS One 10:e0142994
Dumbauld BR, Hosack GR, Bosley KM (2015) Association of juvenile salmon and estuarine fish with intertidal seagrass and oyster aquaculture habitats in a northeast Pacific estuary. Trans Am Fish Soc 144:1091–1110
Elliott M, Whitfield AK, Potter IC, Blaber SJM, Cyrus DP, Nordlie FG, Harrison TD (2007) The guild approach to categorizing estuarine fish assemblages: a global review. Fish Fish 8:241–268
Eschmeyer WN, Herald ES, Hammann H (1983) A field guide to Pacific coast fishes of North America. Houghton Mifflin Company, Boston
Fernandez M, Iribarne O, Armstrong D (1993) Habitat selection by young-of-the-year Dungeness crab Cancer magister and predation risk in intertidal habitats. Mar Ecol Prog Ser 92:171–177
Ferraro SP, Cole FA (2010) Ecological periodic tables for nekton usage of four US Pacific Northwest estuarine habitats. Can J Fish Aquat Sci 67:1957–1967
Ferrell DJ, Bell JD (1991) Differences among assemblages of fish associated with Zostera capricorni and bare sand over a large spatial scale. Mar Ecol Prog Ser 72:15–24
Franco A, Franzoi P, Malavasi S, Riccato F, Torricelli P, Mainardi D (2006) Use of shallow water habitats by fish assemblages in a Mediterranean coastal lagoon. Estuar Coast Shelf Sci 66:67–83
Gross C, Donoghue C, Pruitt C, Trimble AC, Ruesink JL (2017) Taxonomic and functional assessment of mesopredator diversity across an estuarine habitat mosaic. Ecosphere 8(4):e01792. https://doi.org/10.1002/ecs2.1792
Gross C, Donoghue C, Pruitt C, Ruesink JL (2018) Habitat use of eelgrass (Zostera marina) and edges by mesopredators based on capture and camera techniques. Mar Ecol Prog Ser 598:21–33. https://doi.org/10.3354/meps12609
Gross C, Ruesink JL, Pruitt C, Trimble AC, Donoghue CR (2019) Temporal variation in intertidal habitat use by nekton at seasonal and diel scales. J Exp Mar Biol Ecol 516:25–34. https://doi.org/10.1016/j.jembe.2019.04.009
Heck KL, Orth RJ (1980) Seagrass habitats: the role of habitat complexity, competition and predation in structuring associated fish and motile macroinvertebrate assemblages. In: Kennedy VS (ed) Estuarine perspectives. Academic Press, New York, pp 449–464
Heck KL, Able KW, Fahay MP, Roman CT (1989) Fishes and decapods crustaceans of Cape Cod eelgrass meadows—species composition, seasonal abundance patterns and comparison with unvegetated substrates. Estuaries 12:59–65
Heck KL, Hays G, Orth RJ (2003) Critical evaluation of the nursery role hypothesis for seagrass meadows. Mar Ecol Prog Ser 253:123–136
Hensgen GM, Holt GJ, Holt SA, Williams JA, Stunz GW (2014) Landscape pattern influences nekton diversity and abundance in seagrass meadows. Mar Ecol Prog Ser 507:139–152
Hines AH, Haddon AM, Wiechert LA (1990) Guild structure and foraging impact of blue crabs and epibenthic fish in a subestuary of Chesapeake Bay. Mar Ecol Prog Ser 67:105–126
Holsman KK, McDonald PS, Armstrong DA (2006) Intertidal migration and habitat use by subadult Dungeness crab Cancer magister in a NE Pacific estuary. Mar Ecol Prog Ser 308:183–195
Hori M, Suzuki T, Monthum Y, Srisombat T, Tanaka Y, Nakaoka M, Mukai H (2009) High seagrass diversity and canopy-height increase fish diversity and abundance. Mar Biol 156:1447–1458
Horinouchi M (2007) Review of the effects of within-patch scale structural complexity on seagrass fishes. J Exp Mar Biol Ecol 350:111–129
Hosack GR, Dumbauld BR, Ruesink JL, Armstrong DA (2006) Habitat associations of estuarine species: comparisons of intertidal mudflat, seagrass (Zostera marina) and oyster (Crassostrea gigas) habitats. Estuar Coast 29:1150–1160
Hovel KA, Fonseca MS, Myer DL, Kenworthy WJ, Whitfield PE (2002) Effects of seagrass landscape structure, structural complexity and hydrodynamic regime on macrofaunal densities in North Carolina seagrass beds. Mar Ecol Prog Ser 243:11–24
Hughes GW (1985) The comparative ecology and evidence for resource partitioning in two pholidid fishes (Pisces: Pholididae) from southern British Columbia eelgrass beds. Can J Zool 63:76–85
Hughes JE, Deegan LA, Wyda JC, Weaver MJ, Wright A (2002) The effects of eelgrass habitat loss on estuarine fish communities of southern New England. Estuaries 25:235–249
Hughes BB, Levey MD, Brown JA, Fountain MC, Carlisle AB, Litvin SY, Greene CM, Heady WN, Gleason MG (2014) Nursery functions of US West Coast estuaries: the state of knowledge for juveniles of focal invertebrate and fish species. The Nature Conservancy, Arlington
Hyndes GA, Kendrick AJ, MacArthur LD, Stewart E (2003) Differences in the species- and size-composition of fish assemblages in three distinct seagrass habitats with differing plant and meadow structure. Mar Biol 142:1195–1206
Kennedy LA, Juanes F, El-Sabaawi R (2018) Eelgrass as valuable nearshore foraging habitat for juvenile Pacific salmon in the early marine period. Mar Coast Fisher 10:190–203
Kneib RT, Knowlton MK (1993) Stage-structured interactions between seasonal and permanent residents of an estuarine nekton community. Oecologia 103:425–434
Lee H II, Marko K, Hanshumaker M, Folger C, Graham R (2015) User’s guide and metadata to coastal biodiversity risk analysis tool (CBRAT): framework for the systemization of life history and biogeographic information. United States Environmental Protection Agency Western Ecology Division, Newport
Livingston RJ (1984) Trophic response of fishes to habitat variability in coastal seagrass systems. Ecology 65:1258–1275
Mateo Ramirez A, García Raso JE (2012) Temporal changes in the structure of the crustacean decapods assemblages associated with Cymodocea nodosa meadows from the Alboran Sea (Western Mediterranean Sea). Mar Ecol 33:302–316
McDevitt-Irwin JM, Iacarella JC, Baum JK (2016) Reassessing the nursery role of seagrass habitats from temperate to tropical regions: a meta-analysis. Mar Ecol Prog Ser 557:133–143
McPeek KC, McDonald PS, VanBlaricom GR (2015) Aquaculture disturbance impacts the diet but not ecological linkages of a ubiquitous predatory fish. Estuar Coast 38:1520–1534
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2015) Vegan: community ecology package. https://CRAN.R-project.org/package=vegan
Plummer ML, Harvey CJ, Anderson LE, Guerry AD, Ruckelshaus MH (2013) The role of eelgrass in marine community interactions and ecosystem services: results from ecosystem-scale food web models. Ecosystems 16:237–251
Polte P, Asmus H (2006) Influence of seagrass beds (Zostera noltii) on the species composition of juvenile fishes temporarily visiting the intertidal zone of the Wadden Sea. J Sea Res 55:244–252
Potter IC, Tweedley JR, Elliott M, Whitfield AK (2015) The ways in which fish use estuaries: a refinement and expansion of the guild approach. Fish Fish 16:230–239
Reynolds PL, Stachowicz JJ, Hovel K, Bostrom C, Boyer K, Cusson M, Eklof JS, Engel FG, Engelen AH, Eriksson BK, Fodrie FJ, Griffin JN, Hereu CM, Hori M, Hanley TC, Ivanov M, Jorgensen P, Kruschel C, Lee KS, McGlathery K, Moksnes PO, Nakaoka M, O’Connor MI, O’Connor NE, Orth RJ, Rossi F, Ruesink J, Sotka EE, Thormar J, Tomas F, Unsworth RKF, Whalen MA, Duffy JE (2018) Latitude, temperature, and habitat complexity predict predation pressure in eelgrass beds across the Northern Hemisphere. Ecology 99:29–35. https://doi.org/10.1002/ecy.2064
Ribeiro J, Carvalho GM, Gonçalves JMS, Erzini K (2012) Fish assemblages of shallow intertidal habitats of the Ria Formosa lagoon (South Portugal): influence of habitat and season. Mar Ecol Prog Ser 446:259–273
Rotherham D, West RJ (2002) Do different seagrass species support distinct fish communities in south-eastern Australia? Fish Manag Ecol 9:235–248
Ruesink JL, Rowell K (2012) Seasonal effects of clams (Panopea generosa) on eelgrass (Zostera marina) density but not recovery dynamics at an intertidal site. Aquat Conserv 22:712–720
Ruesink JL, Hong JS, Wisehart L, Hacker SD, Dumbauld BR, Hessing-Lewis M, Trimble AC (2010) Congener comparison of native (Zostera marina) and introduced (Z. japonica) eelgrass at multiple scales within a Pacific Northwest estuary. Biol Invasions 12(6):1773–1790
Ryer CH, Orth RJ (1987) Feeding ecology of the northern pipefish, Syngnathus fuscus, in a seagrass community of the lower Chesapeake Bay. Estuaries 10:330–336
Sato M, Horinouchi M, Fujita M, Sano M (2016) Responses of fish assemblage structures to annual and perennial life cycles of seagrass Zostera marina in Lake Hamana, central Japan. Ichthyol Res 63:445–459
Semmens BX (2008) Acoustically derived fine-scale behaviors of juvenile Chinook salmon (Oncorhynchus tshawytscha) associated with intertidal benthic habitats in an estuary. Can J Fish Aquat Sci 65:2053–2062
Smith TM, Hindell JS, Jenkins GP, Connolly RM (2008) Edge effects on fish associated with seagrass and sand patches. Mar Ecol Prog Ser 359:203–213. https://doi.org/10.3354/meps07348
Smith TM, Hindell JS, Jenkins GP, Connolly RM, Keough MJ (2011) Edge effects in patchy seagrass landscapes: the role of predation in determining fish distribution. J Exp Mar Biol Ecol 399:8–16
Spilseth SA, Simenstad CA (2011) Seasonal, diel, and landscape effects on resource partitioning between juvenile Chinook salmon (Oncorhynchus tshawytscha) and Threespine stickleback (Gasterosteus aculeatus) in the Columbia River estuary. Estuar Coasts 34:159–171
Stevens BG, Armstrong DA, Cusimano R (1982) Feeding-habits of the Dungeness crab Cancer magister as determined by the index of relative importance. Mar Biol 72:135–145
Thom RM (1990) Spatial and temporal patterns in plant standing stock and primary production in a temperate seagrass system. Bot Mar 33:497–510
Tuya F, Martín JA, Luque A (2006) Seasonal cycle of a Cymodocea nodosa seagrass meadow and the associated ichthyofauna at Playa Dorada (Lanzarote, Canary Islands, eastern Atlantic). Cienc Mar 32:695–704
Unsworth RKF, Collier CJ, Henderson GM, McKenzie LJ (2012) Tropical seagrass meadows modify seawater carbon chemistry: implications for coral reefs impacted by ocean acidification. Environ Res Lett 7:024026
Valdez SR, Peabody B, Allen B, Blake B, Ruesink JL (2017) Experimental test of oyster restoration within eelgrass. Aquat Conserv 27:578–587. https://doi.org/10.1002/aqc.2722
Vonk JA, Christianen MJA, Stapel J (2010) Abundance, edge effect, and seasonality of fauna in mixed-species seagrass meadows in southwest Sulawesi, Indonesia. Mar Biol Res 6:282–291. https://doi.org/10.1080/17451000903233789
Whitfield AK (2017) The role of seagrass meadows, mangrove forests, salt marshes and reed beds as nursery areas and food sources for fishes in estuaries. Rev Fish Biol Fisher 27:75–110
Wicksten MK (2011) Decapod Crustacea of the Californian and Oregonian Zoogeographic Provinces. Permalink: https://escholarship.org/uc/item/72k9t2dz. Accessed 25 Aug 2018
Włodarska-Kowalczuk M, Jankowska E, Kotwicki L, Balazy P, Unsworth RKF (2014) Evidence of season-dependency in vegetation effects on macrofauna in temperate seagrass meadows (Baltic Sea). PLoS One 9:e100788
Xu Q, Guo D, Ahang P, Ahang X, Li W, Wu Z (2016) Seasonal variation in species composition and abundance of demersal fish and invertebrates in a seagrass natural reserve on the eastern coast of the Shandong Peninsula, China. Chin J Oceanogr Limnol 34:330–341
Acknowledgements
We appreciate field help from Josh Borin, Stephanie Valdez, Joy Polston-Barnes, Elisa Rauschl, Dolores Sare, and Katrina Lassiter.
Funding
This work was funded in part by a Grant from Washington Sea Grant, University of Washington, pursuant to National Oceanic and Atmospheric Administration Award no. NA14OAR4170078. Additional funding came from Washington State Department of Natural Resources (DNR-IAA 16-19). The views expressed herein are those of the authors and do not necessarily reflect the views of NOAA or any of its sub-agencies, or the views of Washington State Department of Natural Resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical statement
Sampling was carried out under Federal (SRP 20047) and state permits (SCP 15-374, 17-246), in accordance with animal care protocols at the University of Washington (IACUC 3363-02).
Data availability
Data are archived at Ruesink J (2018), “Data for: Seagrass increases seasonal variability in nekton but not predation intensity in mid-latitude estuaries”, Mendeley Data, v1 http://dx.doi.org/10.17632/vmvjm6sz69.1
Additional information
Responsible Editor: F. Bulleri.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by R. Best and J. Boada.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ruesink, J.L., Gross, C., Pruitt, C. et al. Habitat structure influences the seasonality of nekton in seagrass. Mar Biol 166, 75 (2019). https://doi.org/10.1007/s00227-019-3519-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3519-z