Abstract
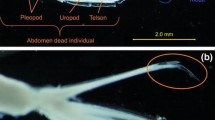
Polar oceans are predicted to be the first marine environments affected by ocean acidification (OA). Thysanoessa inermis is one of the most abundant krill species in northern waters of the Atlantic and a key species in the food web of this ecosystem. Yet, we know very little about potential OA effects on this species. We studied the effects of elevated pCO2 on T. inermis in a laboratory experiment by exposing individuals for 11 weeks to low and high pCO2 (450 and 1200 µatm, respectively, n = 12 per pCO2 treatment). Survival, growth, and moulting frequency was monitored during the experiment, and feeding and oxygen consumption rates (n = 3–5 per pCO2 treatment) were measured at the end of the experiment. No significant effects of high pCO2 on survival, growth, moulting, oxygen consumption, and feeding rate were observed, indicating that T. inermis is tolerant to predicted high OA levels. We also explored physical and chemical properties of waters near the collection area of krill, Rijpfjorden (Svalbard 80° North) during the polar summer (July–August). In situ measurements showed large temperature and salinity gradients from surface to bottom and pCO2 and pH ranged, respectively, 161–417 µatm and 7.99–8.37. Even though substantial spatial variability in pCO2 could be observed, krill in this area is not confronted yet with the investigated high pCO2 levels.






Similar content being viewed by others
References
AMAP (2013) Arctic Monitoring and Assessment Programme—Arctic Ocean Acidification Assessments summary for policy makers, Oslo
Bewick V, Cheek L, Ball J (2004) Statistics review 12: survival analysis. Crit Care 8:389–394. https://doi.org/10.1186/cc2955
Carter HA, Ceballos-Osuna L, Miller NA, Stillman JH (2013) Impact of ocean acidification on metabolism and energetics during early life stages of the intertidal porcelain crab Petrolisthes cinctipes. J Exp Biol 216:1412–1422
Chierici M, Fransson A (2009) CaCO3 saturation in the surface water of the Arctic Ocean: undersaturation in freshwater influenced shelves. Biogeosciences 6:2421–2432. http://www.biogeosciences.net/6/2421/2009
Chierici M, Nomura D, Granskog MA, Kristiansen S, Martma T, Nehrke G (2015) Effect of glacial drainage water on the CO2 system and ocean acidification state in an Arctic tidewater-glacier fjord during two contrasting years. J Geophys Res Oceans 120:2413–2429. https://doi.org/10.1002/2014JC010320
Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chabra A et al (2013) Carbon and other biogeochemical cycles. In: Intergovernmental Panel on Climate Change (ed) Climate change 2013—the physical science basis. Cambridge University Press, Cambridge, pp 465–570
Clayton TD, Byrne RH (1993) Spectrophotometric seawater pH measurements: total hydrogen ion concentration scale calibration of m-cresol purple and at-sea results. Deep Sea Res Part I Oceanogr Res Pap 40:2115–2129. https://doi.org/10.1016/0967-0637(93)90048-8
Cooper HL, Potts DC, Paytan A (2017) Effects of elevated pCO2 on the survival, growth, and moulting of the Pacific krill species, Euphausia pacifica. ICES J Mar Sci 74(4):1005–1012. https://doi.org/10.1093/icesjms/fsw021
Cottier FR, Nilsen F, Inall ME, Gerland S, Tverberg V, Svendsen H (2007) Wintertime warming of an Arctic shelf in response to large-scale atmospheric circulation. Geophys Res Lett 43(10):L10607. https://doi.org/10.1029/2007GL029948
Dalpadado P, Ikeda T (1989) Some observations on moulting, growth and maturation of krill (Thysanoessa inermis) from the Barents Sea. J Plankton Res 11:133–139. https://doi.org/10.1093/plankt/11.1.133
Dalpadado P, Mowbray F (2013) Comparative analysis of feeding ecology of capelin from two shelf ecosystems, off Newfoundland and in the Barents Sea. Prog Oceanogr 114:97–105. https://doi.org/10.1016/j.pocean.2013.05.007
Dalpadado P, Skjoldal HR (1996) Abundance, maturity and growth of the krill species Thysanoessa inermis and T. longicaudata in the Barent Sea. Mar Ecol Prog Ser 144:175–183
Dalpadado P, Ellertsen B, Johannessen S (2008a) Inter-specific variations in distribution, abundance and reproduction strategies of krill and amphipods in the Marginal Ice Zone of the Barents Sea. Deep Sea Res Part II Top Stud Oceanogr 55:2257–2265
Dalpadado P, Yamaguchi A, Ellertsen B, Melle W, Skjoldal HR (2008b) Trophic interactions of macro-zooplankton (krill and amphipods) in the Marginal Ice Zone of the Barents Sea. Deep Sea Res Part II Top Stud Oceanogr 55:2266–2274
Dickson AG, Sabine CL, Christian JR (2007) Guide to best practices for ocean CO2 measurements. PICES Spec Publ 3, British Columbia, p 191
Dolgov AV, Orlova E, Johannesen E, Bogstad B, Rudneva G, Dalpadado P, Mukhina N (2011) Planktivorous fishes. In: Jakobsen T, Ozhigin VK (eds) The Barents Sea. Ecosystem, resources, management. Half a century of Russian-Norwegian Cooperation. Tapir Press, Trondheim, pp 438–454
Drobysheva SS (1994) Euphausiids of the Barents Sea and their role for productivity. PINRO Press, Murmansk (in Russian)
Fransson A, Chierici M, Hop H, Findlay HS, Kristiansen S, Wold A (2016) Late winter- to-summer change in ocean acidification state in Kongsfjorden, with implications for calcifying organisms. Polar Biol. https://doi.org/10.1007/s00300-016-1955-5
Hop H, Falk-Petersen S, Svendsen H, Kwasniewski S, Pavlov V, Pavlova O, Søreide JE (2006) Physical and biological characteristic of the pelagic system across Fram Strait to Kongsfjorden. Prog Oceanogr. https://doi.org/10.1016/j.pocean.2006.099.007
Hopkins CCE, Tande KS, Grønvik S (1984) Ecological investigations of the zooplankton community of Balsfjorden, Northern Norway: an analysis of growth and overwintering tactics in relation to niche and environment in Metridia longa (Lubbock), Calanus finmarchicus (Gunnerus), Thysanoessa inermis (Krøyer) and T. raschi (M. Sars). J Exp Mar Biol Ecol 82:77–99
Huenerlage K, Buchholz F (2015) Thermal limits of krill species from the high-Arctic Kongsfjord (Spitsbergen). Mar Ecol Prog Ser 535:89–98. https://doi.org/10.3354/meps11408
Jonsgård Å (1966) The distribution of Balaenopteridae in the North Atlantic Ocean. In: Norris KS (ed) Whales, dolphins, and porpoises. University of California Press, Berkeley, pp 112–124
Kawaguchi S, Kurihara H, King R, Hale L, Berli T, Robinson JP, Ishida A, Wakita M, Virtue P, Nicole S, Ishimatsu A (2011) Will krill fare well under Southern Ocean acidification? Biol Lett 7:288–291. https://doi.org/10.1098/rsbl.2010.0707
Kawaguchi S, Ishida A, King R, King R, Raymond B, Waller N, Constable A, Nicol S, Wakita M, Ishimatsu A (2013) Risk maps for Antarctic krill under projected Southern Ocean acidification. Nat Clim Chang 3:843–847. https://doi.org/10.1038/nclimate1937
Kiørboe T (2013) Zooplankton body composition. Limnol Oceanogr 58:1843–1850
Kroeker KJ, Kordas RL, Crim R, Singh GG et al (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Chang Biol 19:1884–1896. https://doi.org/10.1111/gcb.12179
Kurihara H (2008) Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar Ecol Prog Ser 373:275–284. https://doi.org/10.3354/meps07802
Long CW, Swiney KM, Harris C, Page HN, Foy RJ (2013) Effects of ocean acidification on juvenile red king crab (Paralithodes camtschaticus) and tanner crab (Chionoecetes bairdi) growth conditions, calcification, and survival. PLoS One 8(4):e60959. https://doi.org/10.1371/journal.pone.0060959
Marin V, Huntley ME, Frost B (1986) Measuring feeding rates of pelagic herbivores: analysis of experimental design and methods. Mar Biol 93:49–58. https://doi.org/10.1007/BF00428654
Marin V, Huntley ME, Frost B (1987) Corrigendum. Mar Biol 95:156
Marinovic B, Mangel M (1999) Krill can shrink as an ecological adaptation to temporarily unfavorable environments. Ecol Lett 2:338–343
Mclaskey AK, Keister JE, McElhany P, Olson BM, Busch D, Maher M, Winans AK (2016) Development of Euphausia pacifica (krill) larvae is impaired under pCO2 levels currently observed in the Northeast Pacific. Mar Ecol Prog Ser 555:65–78
Pierrot D, Lewis E, Wallace DWR (2006) MS Excel Program developed for CO2 system calculations. ORNL/CDIAC-105a. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge. https://doi.org/10.3334/cdiac/otg.co2sys_xls_cdiac105a
R Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Riahi K, Rao S, Krey V, Cho C, Chirkov V, Fischer G, Kindermann G, Nakicenovic N, Rafaj P (2011) A scenario of comparatively high greenhouse gas emissions. Clim Change 109:33–57
Saba GK, Schofield O, Torres JJ, Ombres EH, Steinberg DK (2012) Increased feeding and nutrient excretion of adult Antarctic krill, Euphausia superba, exposed to enhanced carbon dioxide (CO2). PLoS One 7:e52224. https://doi.org/10.1371/journal.pone.0052224
Sabine CL, Feely RA, Gruber N, Key RM, Lee K, Bullister JL, Wanninkhof R, Wong CS, Wallace DWR, Tilbrook B, Millero FJ, Peng TH, Kozyr A, Ono T, Rios AF (2004) The oceanic sink for anthropogenic CO2. Science 305:367–371. https://doi.org/10.1126/science.1097403
Skaug HJ, Gjøsæter H, Haug T, Nilssen KT, Lindstrøm U (1997) Do mink whales (Balaenoptera acutorostrata) exhibit particular prey preferences? J North Atl Fish Sci 22:91–104
Skogen MD, Olsen A, Børsheim KY, Sandø AB, Skjelvan I (2014) Modelling ocean acidification in the Nordic and Barents Seas in present and future climate. J Mar Syst 131:10–20
Small DP, Calosi P, Boothroyd D, Boothroyd D, Widdicombe S, Spicer JI (2016) The sensitivity of the early benthic juvenile stage of the European lobster Homarus gammarus (L.) to elevated pCO2 and temperature. Mar Biol 163:53. https://doi.org/10.1007/s00227-016-2834-x
Sperfeld E, Mangor-Jensen A, Dalpadado P (2014) Effect of increasing sea water pCO2 on the northern Atlantic krill species Nyctiphanes couchii. Mar Biol 161:2359–2370. https://doi.org/10.1007/s00227-014-2511-x
Sperfeld E, Mangor-Jensen A, Dalpadado P (2017) Effects of increasing pCO2 on life history traits and feeding on the littoral mysid Praunus flexuosus. Mar Biol 164:173. https://doi.org/10.1007/s00227-017-3203-0
Takahashi T, Olafsson J, Goddard JG, Chipman DW, Sutherland SC (1993) Seasonal variation of CO2 and nutrients in the high-latitude surface oceans: a comparative study. Glob Biochem Cycles 7:843–878
Therneau T (2012) A package for survival analysis in S. R package version 2.36-14
Thor P, Oliva EO (2015) Ocean acidification elicits different energetic responses in an Arctic and a boreal population of the copepod Pseudocalanus acuspes. Mar Biol 162:799–807
Venello TA, Calosi P, Turner LM, Findlay HS (2017) Overwintering individuals of the Arctic krill Thysanoessa inermis appear tolerant to short-term exposure to low pH condition. Polar Biol. https://doi.org/10.1007/s00300-017-2194-0
Walther K, Sartoris FJ, Pὂrtner HO (2009) Impact of anthropogenic ocean acidification on thermal tolerance of the spider crab Hyas araneus. Biogeosciences 6:2207–2215
Wang CL, Shi S, Gerland MA, Granskog A, Renner HH, Li Z, Hansen E, Martma T (2013) Spring sea-ice evolution in Rijpfjorden (80°N), Svalbard, from in situ measurements and ice mass-balance buoy (IMB) data. Ann Glaciol. https://doi.org/10.3189/2013AoG62A135
Whiteley NM (2011) Physiological and ecological responses of crustaceans to ocean acidification. Mar Ecol Prog Ser 430:257–271. https://doi.org/10.3354/meps09185
Wittmann AC, Pörtner HO (2013) Sensitivies of extant animal taxa to ocean acidification. Nat Clim Change 3:995–1001. https://doi.org/10.1038/ncimate1982
Acknowledgements
We are grateful to the crew and scientists on R/V Lance for collecting and shipping krill and to the technical staff at the Austevoll Research Station of the Institute of Marine Research for making this experiment possible. This work was supported by funds from the Norwegian Ministry of Fisheries. Parts of the work were supported by the Flagship research program “Ocean acidification and ecosystem effects in Northern waters” within the FRAM-High North Research Centre for Climate and the Environment. E. Sperfeld acknowledges the International IGB Fellowship Program of the Leibniz-Institute of Freshwater Ecology and Inland Fisheries (IGB, Berlin, Germany) for partial financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare they have no conflict of interest.
Ethical standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: A. Atkinson.
Reviewed by Undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Opstad, I., Mangor-Jensen, A., Sperfeld, E. et al. Effects of high pCO2 on the northern krill Thysanoessa inermis in relation to carbonate chemistry of its collection area, Rijpfjorden. Mar Biol 165, 116 (2018). https://doi.org/10.1007/s00227-018-3370-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-018-3370-7