Abstract
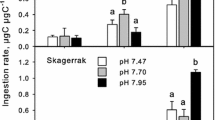
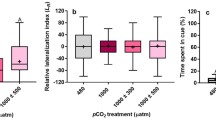
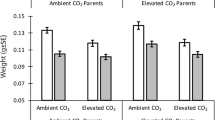
As the oceans acidify, marine invertebrates will experience physiological and behavioural changes that may alter how predators interact with their prey. This study assessed whether ocean acidification alters the predatory whelk Tenguella marginalba, their prey, the Pacific oyster, Crassostrea gigas, and their interactions. Oysters and whelks were exposed separately to ambient or elevated pCO2 for 6 weeks, after which, a reciprocal cross design was used to expose oysters and whelks together to ambient and elevated pCO2. Both T. marginalba and C. gigas were measured for growth, shell morphology, shell compression strength and metabolic rate. The rate at which whelks consumed oysters was also measured. We found C. gigas had weaker shells and greater SMR at elevated pCO2, but lowered its SMR when held at ambient pCO2 with T. marginalba. T. marginalba had a greater SMR and consumed more C. gigas when both the predator and prey were held at elevated pCO2. We also tested whether C. gigas responses to predator chemical cues were altered by ocean acidification. C. gigas lowered its metabolic rate in response to predator cues at ambient, but not elevated pCO2. We conclude that elevated pCO2 may increase the energy requirements of predators, as they attempt to maintain homoeostasis. Furthermore, elevated pCO2 may also alter the morphology and increase the visibility of prey. Whether the consequence of this will be a sustained increase in consumption by the predator is less certain as molluscs acclimate and the dynamics of other organisms in marine ecosystems are also altered.





Similar content being viewed by others
References
Bibby R, Cleall-Harding P, Rundle S, Widdicombe S, Spicer J (2007) Ocean acidification disrupts induced defences in the intertidal gastropod Littorina littorea. Biol Lett 3:699–701
Bibby R, Widdicombe S, Parry H, Spicer J, Pipe R (2008) Effect of ocean acidification on the immune response of the blue mussel, Mytilus edulis. Aquat Biol 2:67–74
Blake JW (1960) Oxygen consumption of bivalve prey and their attractiveness to the gastropod, Urosalpinx cineera. Limnol Oceanogr 5:273–280
Bourdeau PE (2009) Prioritized phenotypic responses to combined predators in a marine snail. Ecology 90:1659–1669
Bourdeau PE (2012) Intraspecific trait cospecialization of constitutive and inducible morphological defences in a marine snail from habitats with different predation risk. J Anim Ecol 81:849–858
Chivers DP, Smith RJF (1998) Chemical alarm signalling in aquatic predator–prey systems: a review and prospectus. Ecoscience 5:338–352
Cummings V, Hewitt J, Van Rooyen A, Currie K, Beard S, Thrush S, Norkko J, Barr N, Heath P, Halliday NJ (2011) Ocean acidification at high latitudes: potential effects on functioning of the Antarctic bivalve Laternula elliptica. PLoS One 6:e16069
Currey J (1976) Further studies on the mechanical properties of mollusc shell material. J Zool 180:445–453
Dupont ST, Mercurio M, Giacoletti A, Rinaldi A, Mirto S, D’Acquisto L, Sabatino MA, Sara G (2015) Functional consequences of prey acclimation to ocean acidification for the prey and its predator. PeerJ PrePrints 3:e1792
Ferrari MC, Wisenden BD, Chivers DP (2010) Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus The present review is one in the special series of reviews on animal–plant interactions. Can J Zool 88:698–724
Ferrari MC, McCormick MI, Munday PL, Meekan MG, Dixson DL, Lonnstedt Ö, Chivers DP (2011) Putting prey and predator into the CO2 equation–qualitative and quantitative effects of ocean acidification on predator–prey interactions. Ecol Lett 14:1143–1148
Gran G (1952) Determination of the equivalence point in potentiometric titrations. Part II. Anal 77:661–671
Green MA, Waldbusser GG, Reilly SL, Emerson K (2009) Death by dissolution: sediment saturation state as a mortality factor for juvenile bivalves. Limnol Oceanogr 54:1037–1047
IPCC (2013) Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the intergovernmental panel on climate change, Cambridge, United Kingdom and New York, NY, USA
Jacobsen HP, Stabell OB (2004) Antipredator behaviour mediated by chemical cues: the role of conspecific alarm signalling and predator labelling in the avoidance response of a marine gastropod. Oikos 104:43–50
Jellison BM, Ninokawa AT, Hill TM, Sanford E, Gaylord B (2016) Ocean acidification alters the response of intertidal snails to a key sea star predator. In: Proceedings of the Royal Society B. The Royal Society, p 20160890
Leonard GH, Bertness MD, Yund PO (1999) Crab predation, waterborne cues, and inducible defenses in the blue mussel, Mytilus edulis. Ecology 80:1–14
Lesser MP (2016) Climate change stressors cause metabolic depression in the blue mussel, Mytilus edulis, from the Gulf of Maine. Limnol Oceanogr 61:1705–1717
Lewis E, Wallace D, Allison LJ (1998) Program developed for CO2 system calculations: Carbon Dioxide Information Analysis Center, managed by Lockheed Martin Energy Research Corporation for the US Department of Energy Tennessee
Li L, Lu W, Sui Y, Wang Y, Gul Y, Dupont S (2015) Conflicting effects of predator cue and ocean acidification on the mussel Mytilus coruscus byssus production. J Shellfish Res 34:393–400
Lischka S, Buedenbender J, Boxhammer T, Riebesell U (2010) Impact of ocean acidification and elevated temperatures on early juveniles of the polar shelled pteropod Limacina helicina: mortality, shell degradation, and shell growth. Biogeosci Disc 7:8177–8214
Liu W, He M (2012) Effects of ocean acidification on the metabolic rates of three species of bivalve from southern coast of China. Chin J Oceanol Limnol 30:206–211
Lord JP, Whitlatch RB (2012) Inducible defenses in the eastern oyster Crassostrea virginica Gmelin in response to the presence of the predatory oyster drill Urosalpinx cinerea say in long island sound. Mar Biol 159:1177–1182
Manríquez PH, Jara ME, Mardones ML, Navarro JM, Torres R, Lardies MA, Vargas CA, Duarte C, Widdicombe S, Salisbury J (2013) Ocean acidification disrupts prey responses to predator cues but not net prey shell growth in Concholepas concholepas (loco). PLoS One 8:e68643
Manríquez PH, Jara ME, Seguel ME, Torres R, Alarcon E, Lee MR (2016) Ocean acidification and increased temperature have both positive and negative effects on early ontogenetic traits of a rocky shore keystone predator species. PLoS One 11:e0151920
Mehrbach C, Culberson C, Hawley J, Pytkowicz R (1973) Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr 18:897–907
Melzner F, Stange P, Trübenbach K, Thomsen J, Casties I, Panknin U, Gorb SN, Gutowska MA (2011) Food supply and seawater pCO2 impact calcification and internal shell dissolution in the Blue mussel Mytilus edulis. PLoS One 6:e24223
Michaelidis B, Ouzounis C, Paleras A, Pörtner HO (2005) Effects of long-term moderate hypercapnia on acid-base balance and growth rate in marine mussels Mytilus galloprovincialis. Mar Ecol Prog Ser 293:109–118
Morgan SG, Gravem SA, Lipus AC, Grabiel M, Miner BG (2016) Trait-mediated indirect interactions among residents of rocky shore tidepools. Mar Ecol Prog Ser 552:31–46
Navarro JM, Torres R, Acuña K, Duarte C, Manriquez PH, Lardies M, Lagos NA, Vargas C, Aguilera V (2012) Impact of medium-term exposure to elevated pCO2 levels on the physiological energetics of the mussel Mytilus chilensis. Chemosphere 90:1242–1248
Parker LM, Ross PM, O’Connor WA (2011) Populations of the Sydney rock oyster, Saccostrea glomerata, vary in response to ocean acidification. Mar Biol 158:689–697
Parker LM, Ross PM, O’Connor WA, Borysko L, Raftos DA, Pörtner HO (2012) Adult exposure influences offspring response to ocean acidification in oysters. Glob Change Biol 18:82–92
Parker LM, Ross PM, O’Connor WA, Pörtner HO, Scanes E, Wright JM (2013) Predicting the response of molluscs to the impact of ocean acidification. Biology 2:651–692
Pörtner HO, Langenbuch M, Reipschläger A (2004) Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. J Oceanogr 60:705–718
Pratt D (1974) Attraction to prey and stimulus to attack in the predatory gastropod Urosalpinx cinerea. Mar Biol 27:37–45
Queirós AM, Fernandes JA, Faulwetter S, Nunes J, Rastrick SP, Mieszkowska N, Artioli Y, Yool A, Calosi P, Arvanitidis C (2015) Scaling up experimental ocean acidification and warming research: from individuals to the ecosystem. Glob Change Biol 21:130–143
Range P, Chícharo M, Ben-Hamadou R, Piló D, Matias D, Joaquim S, Oliveira A, Chícharo L (2011) Calcification, growth and mortality of juvenile clams Ruditapes decussatus under increased pCO2 and reduced pH: variable responses to ocean acidification at local scales? J Exp Mar Biol Ecol 396:177–184
Riebesell U, Fabry VJ, Hansson L, Gattuso J-P (2010) Guide to best practices for ocean acidification research and data reporting. Publications Office of the European Union, Luxembourg
Sanford E, Gaylord B, Hettinger A, Lenz EA, Meyer K, Hill TM (2014) Ocean acidification increases the vulnerability of native oysters to predation by invasive snails. Proc R Soc B 281:20132681
Scanes E, Parker LM, O'Connor WA, Stapp LS, Ross PM (2017) Intertidal oysters reach their physiological limit in a future high-CO2 world. J Exp Biol 220(5):765–774
Schalkhausser B, Bock C, Stemmer K, Brey T, Pörtner H-O, Lannig G (2013) Impact of ocean acidification on escape performance of the king scallop, Pecten maximus, from Norway. Mar Biol 160:1995–2006
Schoeppner NM, Relyea RA (2005) Damage, digestion, and defence: the roles of alarm cues and kairomones for inducing prey defences. Ecol Lett 8:505–512
Smith L, Jennings J (2000) Induced defensive responses by the bivalve Mytilus edulis to predators with different attack modes. Mar Biol 136:461–469
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. W. H. Freeman and Co., New York
Sui Y, Hu M, Huang X, Wang Y, Lu W (2015) Anti-predatory responses of the thick shell mussel Mytilus coruscus exposed to seawater acidification and hypoxia. Mar Environ Res 109:159–167
R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Trussell GC, Nicklin MO (2002) Cue sensitivity, inducible defense, and trade-offs in a marine snail. Ecology 83:1635–1647
Underwood A, Chapman M, Richards S (2002) GMAV-5 for Windows. An analysis of variance programme. In: Centre for research on ecological impacts of coastal cities. Marine Ecology Laboratories, University of Sydney, Australia
Wang Y, Hu M, Cheung SG, Shin PK, Lu W, Li J (2013) Antipredatory responses of Perna viridis (Linnaeus, 1758) under acute hypoxia and low salinity. J Mollus Stud 79:42–50
Watson S-A, Lefevre S, McCormick MI, Domenici P, Nilsson GE, Munday PL (2014) Marine mollusc predator-escape behaviour altered by near-future carbon dioxide levels. Proc R Soc B 281:20132377
Winer BJ, Brown DR, Michels KM (1971) Statistical principles in experimental design. McGraw-Hill, New York
Wright JM, Parker LM, O’Connor WA, Williams M, Kube P, Ross PM (2014) Populations of Pacific Oysters Crassostrea gigas respond variably to elevated CO2 and predation by Morula marginalba. Biol Bull 226:269–281
Wright JM, Parker LM, O’Connor WA, Ross PM (2017) Predation by the endemic whelk Tenguella marginalba (Blainville, 1832) on the invasive Pacific oyster Crassostrea gigas (Thunberg, 1793). J Mollusc Res. https://doi.org/10.1080/13235818.2017.1420397
Zuschin M, Stanton J (2001) Experimental measurement of shell strength and its taphonomic interpretation. Palaios 16:161–170
Acknowledgements
We wish to acknowledge the support of the New South Wales Department of Primary Industries. We would also like to acknowledge the Institute of Infrastructure and Engineering at Western Sydney University for the provision of mechanical facilities. The authors would like to thank the staff and volunteers at the Port Stephens Fisheries Institute and the School of Science and Health at Western Sydney University, especially Victoria Cole, Stephan O’Connor, Kyle Johnston, Justin Kelly, Brandt Archer, Andrew Parnell, Lyne Foulkes, Linda Westmoreland and the Hawkesbury technical team. We wish to thank the reviewers for their comments which have helped to improve this manuscript. Support for the writing of this manuscript has been provided by the School of Life and Environmental Sciences at the University of Sydney.
Funding
JM Wright was supported by an Australian Postgraduate Award and would like to thank Western Sydney University and the University of Sydney for funding this research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare they have no competing interests. All animals have been sampled and treated according to the national legislation and all required permissions have been obtained.
Additional information
Responsible Editor: G. Chapman.
Reviewed by K. Benkendorff, A. Jackson, J. Ruesink and an undisclosed expert.
Rights and permissions
About this article
Cite this article
Wright, J.M., Parker, L.M., O’Connor, W.A. et al. Ocean acidification affects both the predator and prey to alter interactions between the oyster Crassostrea gigas (Thunberg, 1793) and the whelk Tenguella marginalba (Blainville, 1832). Mar Biol 165, 46 (2018). https://doi.org/10.1007/s00227-018-3302-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-018-3302-6