Abstract
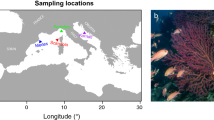
Changes in global climate patterns are affecting marine ecosystems, challenging species’ environmental tolerances, and driving shifts in their distributions. In the Baltic Sea, a brackish water body with low biodiversity, the isopod Idotea balthica is a key herbivore species that has a strong top–down effect on habitat-forming macrophytes. Our aim is to understand how the predicted future combination of hyposalinity and warming will affect the survival of this mesograzer throughout the Baltic Sea. By conducting a manipulative aquarium experiment, we simulated future conditions and measured the survival, at different spatial scales, of replicated populations from the entrance, central, and marginal Baltic Sea regions. Overall, the survival rate was strongly affected by the predicted future combination of hyposalinity and warming, but the intensity of the impact varied both among and within regions. Populations from the marginal Baltic Sea responded negatively to climate change. Populations within the entrance varied in their survival responses, with the geographic variation suggesting the existence of spatially distributed genetic variation in tolerance to climate change. In summary, the future combination of hyposalinity and warming is likely to induce a southward shift in the distribution of I. balthica in the northeast marginal region of the Baltic Sea. However, the geographic variation in tolerance shown by the entrance populations indicates that, for this Baltic region, the species may contain the potential for future adaptive responses in tolerance to climate change.




Similar content being viewed by others
References
Allison PD (2010) Survival analysis using the SAS system: a practical quide, 2nd edn. SAS. Institute Inc., Gary
Altieri AH, Gedan KB (2015) Climate change and dead zones. Glob Chang Biol 21:1395–1406
Antonov JI (2002) Steric sea level variations during 1957–1994: importance of salinity. J Geophys Res 107:1–8
Bell G, Gonzalez A (2009) Evolutionary rescue can prevent extinction following environmental change. Ecol Lett 12:942–948
Bonsdorff E, Andersson A, Elmgren R (2015) Baltic Sea ecosystem-based management under climate change: integrating social and ecological perspectives. Ambio 44:333–334
Boyer TP, Levitus S, Antonov JI, Locarnini RA, Garcia HE (2005) Linear trends in salinity for the World Ocean, 1955–1998. Geophys Res Lett 32:1–4
Brierley AS, Kingsford MJ (2009) Impacts of climate change on marine organisms and ecosystems. Curr Biol 19:602–614
Bulnheim HP (1974) Respiratory metabolism of Idotea baltica (Crustacea, Isopoda) in relation to environmental variables, acclimation processes and moulting. Helgoländer Meeresunters 26:464–480
Coma R, Ribes M, Serrano E, Jimenez E, Salat J, Pascual J (2009) Global warming-enhanced stratification and mass mortality events in the Mediterranean. Proc Nat Acad Sci USA 106:6176–6181
Crain C, Francisco S, National B, Resear E (2008) Interactive and cumulative effects of multiple stressors in marine systems. Ecol Lett 11:1304–1315
Crispo E (2008) Modifying effects of phenotypic plasticity on interactions among natural selection, adaptation and gene flow. J Evol Biol 21:1460–1469
D’angelo C, Hume BC, Burt J, Smith EG, Achterberg EP, Wiedenmann J (2015) Local adaptation constrains the distribution potential of heat-tolerant Symbiodinium from the Persian/Arabian Gulf. ISME J 9:2551–2560
Defaveri J, Merila J (2014) Local adaptation to salinity in the three-spined stickleback? J Evol Biol 27:290–302
Dunn DW, Sumner JP, Goulson D (2005) The benefits of multiple mating to female seaweed flies, Coelopa frigida (Diptera: Coelpidae). Behav Ecol Sociobiol 58:128–135
Feistel R, Weinreben S, Wolf H, Seitz S, Spitzer P, Adel B, Nausch G, Schneider B, Wright DG (2010) Density and absolute salinity of the Baltic Sea 2006–2009. Ocean Sci 7:3–24
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251
Goecker ME, Kåll SE (2003) Grazing preferences of marine isopods and amphipods on three prominent algal species of the Baltic Sea. J Sea Res 50:309–314
Gunnarsson K, Berglund A (2012) The brown alga Fucus radicans suffers heavy grazing by the isopod Idotea baltica. Mar Biol Res 8:87–89
Gutow L, Petersen I, Bartl K, Huenerlage K (2016) Marine meso-herbivore consumption scales faster with temperature than seaweed primary production. J Exp Mar Bio Ecol 477:80–85
Haahtela I (1984) A hypothesis of the decline of the bladder wrack Fucus vesiculosus L. in SW Finland in 1975–1981. Limnologica 15:345–350
Haavisto F, Jormalainen V (2014) Seasonality elicits herbivores’ escape from trophic control and favors induced resistance in a temperate macroalga. Ecology 95:3035–3045
Harley CDG, Hughes AR, Hultgren KM, Miner BG, Sorte CJB, Thornber CS, Rodriguez LF, Tomanek L, Williams SL (2006) The impacts of climate change in coastal marine systems. Ecol Lett 9:228–241
Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, Hoffman EE, Lipp EK, Osterhaus ADME, Overstreet RM, Porter JW (1999) Emerging marine diseases: climate links and anthropogenic factors. Science 285:1505–1510
Harvell D, Aronson R, Baron N, Connell J, Dobson A, Ellner S, Gerber L, Kim K, Kuris A, McCallum H, Lafferty K (2004) The rising tide of ocean diseases: unsolved problems and research priorities. Front Ecol Environ 2:375–382
Harvey BP, Al-janabi B, Broszeit S, Cioffi R, Kumar A, Aranguren-Gassis M, Bailey A, Green L, Gsottbauer CM, Hall EF, Lechler M, Mancuso FP, Pereira CO, Ricevuto E, Schram JB, Stapp LS, Stenberg S, Rosa LTS (2014) Evolution of marine organisms under climate change at different levels of biological organisation. Water 6:3545–3574
Helmuth B, Mieszkowska N, Moore P, Hawkins SJ (2006) Living on the edge of two changing worlds: forecasting the responses of rocky intertidal ecosystems to climate change. Annu Rev Ecol Evol Syst 37:373–404
Hoegh-Guldberg O, Mumby PJ, Hooten A, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Hoffmann AA, Sgrò CM (2011) Climate change and evolutionary adaptation. Nature 470:479–485
Hørlyck V (1973) The osmoregulatory ability in three species of the genus Idotea (Isopoda, crustacea). Ophelia 12:129–140
Jansen KP (1970) Effect of temperature and salinity on survival and reproduction in Baltic populations of Sphaeroma hookeri Leach, 1814 and S. rugicauda Leach, 1814 (Isopoda). Ophelia 7:177–184
Johannesson K, André C (2006) Life on the margin: genetic isolation and diversity loss in a peripheral marine ecosystem, the Baltic Sea. Mol Ecol 15:2013–2029
Johannesson K, Smolarz K, Grahn M, André C (2011) The future of Baltic Sea populations: local extinction or evolutionary rescue? Ambio 40:179–190
Jormalainen V, Ramsay T (2009) Resistance of the brown alga Fucus vesiculosus to herbivory. Oikos 118:713–722
Jormalainen V, Tuomi J (1989a) Sexual differences in habitat selection and activity of the colour polymorphic isopod Idotea baltica. Anim Behav 38:576–585
Jormalainen V, Tuomi J (1989b) Reproductive ecology of the isopod Idotea balthica (Pallas) in the Northern Baltic. Ophelia 3:213–223
Jormalainen V, Honkanen T, Makinen A, Hemmi A, Vesakoski O (2001) Why does herbivore sex matter? Sexual differences in utilization of Fucus vesiculosus by the isopod Idotea baltica. Oikos 93:77–86
Jormalainen V, Honkanen T, Vesakoski O, Koivikko R (2005) Polar extracts of the brown alga Fucus vesiculosus (L.) reduce assimilation efficiency but do not deter the herbivorous isopod Idotea baltica (Pallas). J Exp Mar Bio Ecol 317:143–157
Kalbfleisch JD, Prentice RL (1980) Statistical analysis of failure time data. Wiley, New York
Kangas P, Autio H, Hällfors G, Luther H, Niemi Å, Salemaa H (1982) A general model of the decline of Fucus vesiculosus at Tvärminne, south coast of Finland in 1977–81. Acta Bot Fenn 118:1–27
Kawecki TJ, Ebert D (2004) Conceptual issues in local adaptation. Ecol Lett 7:1225–1241
Knights AM, Firth LB, Russell BD (2017) Ecological responses to environmental change in marine systems. J Mar Biol Ecol 492:1–140
Lamichhaney S, Barrio AM, Rafati N (2012) Population-scale sequencing reveals genetic differentiation due to local adaptation in Atlantic herring. Proc Natl Acad Sci USA 109:19345–19350
Łapucki T, Normant M (2008) Physiological responses to salinity changes of the isopod Idotea chelipes from the Baltic brackish waters. Comp Biochem Physiol: Mol Integr Physiol 149:299–305
Lass HU, Matthäus U (2008) General oceanography of the Baltic Sea. In: Feistel R, Nausch G, Wasmund N (eds) State and evolution of the Baltic Sea, 1952–2005. Wiley, Hoboken, pp 5–44
Leidenberger S, Harding K, Jonsson PR (2012) Ecology and distribution of the isopod genus Idotea in the Baltic Sea: key species in a changing environment. J Crustac Biol 32:359–381
Lenoir J, Svenning JC (2015) Climate-related range shifts—a global multidimensional synthesis and new research directions. Ecography 38:15–28
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for mixed models, 2nd edn. SAS Institute, Cary
Meier HEM, Eilola K (2011) Future projections of ecological patterns in the Baltic Sea. SMHI Reports, Oceanografi 107
Meier HEM, Hordoir R, Andersson HC, Dieterich C, Eilola K, Gustafsson BG, Höglund A, Schimanke S (2012) Modeling the combined impact of changing climate and changing nutrient loads on the Baltic Sea environment in an ensemble of transient simulations for 1961–2099. Clim Dyn 39:2421–2441
Muir AP, Nunes FL, Dubois SF, Pernet F (2016) Lipid remodelling in the reef-building honeycomb worm, Sabellaria alveolata, reflects acclimation and local adaptation to temperature. Sci Rep 6:35669
Nilsson J, Engkvist R, Persson LE (2004) Long-term decline and recent recovery of Fucus populations along the rocky shores of southeast Sweden, Baltic Sea. Aquat Ecol 38:587–598
Normant M, Lamprecht I (2006) Does scope for growth change as a result of salinity stress in the amphipod Gammarus oceanicus? J Exp Mar Bio Ecol 334:158–163
Orav-Kotta H, Kotta J (2004) Food and habitat choice of the isopod Idotea baltica in the northeastern Baltic Sea. Hydrobiologia 514:79–85
Pauls SU, Nowak C, Bálint M, Pfenninger M (2013) The impact of global climate change on genetic diversity within populations and species. Mol Ecol 22:925–946
Poloczanska ES, Babcock RC, Butler A, Hobday AJ, Hoegh-Guldberg O, Kunz TJ, Matear R, Milton DA, Okey TA, Richardson AJ (2007) Climate change and Australian marine life. Ocean Mar Biol Annu Rev 45:407–478
Pörtner HO (2001) Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88:137–146
Postel U, Becker W, Brandt A, Luck-Kopp S, Riestenpatt S, Weihrauch D, Siebers D (2000) Active osmoregulatory ion uptake across the pleopods of the isopod Idotea baltica (Pallas): electrophysiological measurements on isolated split endo- and exopodites mounted in a micro-ussing chamber. J Exp Biol 203:1141–1152
Przeslawski R, Ahyong S, Byrne M, Wörheide G, Hutchings P (2008) Beyond corals and fish: the effects of climate change on noncoral benthic invertebrates of tropical reefs. Glob Chang Biol 14:2773–2795
Qiu J-W, Qian P-Y (1999) Tolerance of the barnacle Balanus amphitrite to salinity and temperature stress: effects of previous experience. Mar Ecol Prog Ser 188:123–132
Ravanko O (1969) Benthic algae as food for some invertebrates in the inner part of the Baltic. Limnologica 7:203–205
Rivera-Ingraham GA, Lignot JH (2017) Osmoregulation, bioenergetics and oxidative stress in coastal marine invertebrates: raising the questions for future research. J Exp Biol 220:1749–1760
Rosenberg NE, Sirkus L (2011) Survival analysis using SAS: a practical guide. Second Edition By Paul D. Allison. Am J Epidemiol 174:503–504
Roth O, Kurtz J, Reusch TBH (2010) A summer heat wave decreases the immunocompetence of the mesograzer, Idotea balthica. Mar Biol 157:1605–1611
Saada G, Nicastro KR, Jacinto R, McQuaid CD, Serrão EA, Pearson GA, Zardi GI, Schoeman D (2016) Taking the heat: distinct vulnerability to thermal stress of central and threatened peripheral lineages of a marine macroalga. Divers Distrib 22:1060–1068
Salemaa H (1979) Ecology of Idotea spp (Isopoda) in the northern Baltic. Ophelia 18:133–150
Salemaa H (1987) Herbivory and microhabitat preferences of Idotea spp. (Isopoda) in the northern Baltic Sea. Ophelia 27:1–15
Salomon M, Buchholz F (2000) Effects of temperature on the respiration rates and the kinetics of citrate synthase in two species of Idotea (Isopoda, Crustacea). Comp Biochem Physiol: B Biochem Mol Biol 125:71–81
Sanford E, Kelly MW (2011) Local adaptation in marine invertebrates. Annu Rev Mar Sci 3:509–535
Slatkin M (1985) Gene flow in natural populations. Annu Rev Ecol Syst 16:393–430
Strong KW, Daborn GR (1980) The influence of temperature on energy budget variables, body size, and seasonal occurrence of the isopod Idotea balhtica (Pallas). Can J Zool 58:1992–1996
Torres G, Giménez L, Anger K (2011) Growth, tolerance to low-salinity, and osmoregulation in decapod crustacean larvae. Aquat Biol 12:249–260
Valladares F, Matesanz S, Araujo MB, Balaguer L, Benito-Garzon M, Cornwell WK, Gianoli E, Guilhaumon F, van Kleunen M, Naya D, Nicotra AB, Poorter H, Zavala MA (2014) The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol Lett 17:1351–1364
Vergés A, Steinberg PD, Hay ME, Poore AGB, Campbell AH, Ballesteros E, Heck KL, Booth DJ, Coleman MA, Feary DA, Figueira W, Langlois T, Marzinelli EM, Mizerek T, Mumby PJ, Nakamura Y, Roughan M, van Sebille E, Sen Gupta A, Smale DA, Tomas F, Wernberg T, Wilson SK (2014) The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc R Soc B 281:1–10
Wernberg T, Smale DA, Thomsen MS (2012) A decade of climate change experiments on marine organisms: procedures, patterns and problems. Glob Chang Biol 18:1491–1498
Werner FJ, Graiff A, Matthiessen B (2015) Temperature effects on seaweed-sustaining top-down control vary with season. Oecologia 180:889–901
Wood HL, Nylund G, Eriksson SP (2014) Physiological plasticity is key to the presence of the isopod Idotea balthica (Pallas) in the Baltic Sea. J Sea Res 85:255–262
Wrange AL, André C, Lundh T, Lind U, Blomberg A, Jonsson PJ, Havenhand JN (2014) Importance of plasticity and local adaptation for coping with changing salinity in coastal areas: a test case with barnacles in the Baltic Sea. BMC Evol Biol 14:156
Acknowledgments
We thank S. Mikkonen, K. Riipinen, and E. Rothäusler for valuable assistance during sampling and laboratory work. We are also thankful to the Archipelago Research Institute of the University of Turku for the use of their facilities and logistical help.
Author information
Authors and Affiliations
Contributions
VJ, LR and IM conceived and designed the experiment. LR, IM and JS performed the experiment. LR and IM analysed the data. LR led the writing of the manuscript; all authors contributed to the text.
Corresponding author
Ethics declarations
Funding
This study was funded by BONUS, the EU joint Baltic Sea research and development programme, Project BAMBI and the Academy of Finland Grant decision number 273623.
Conflict of interest
all authors declare that there is no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: F. Bulleri.
Reviewed by undisclosed experts.
Rights and permissions
About this article
Cite this article
Rugiu, L., Manninen, I., Sjöroos, J. et al. Variations in tolerance to climate change in a key littoral herbivore. Mar Biol 165, 18 (2018). https://doi.org/10.1007/s00227-017-3275-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3275-x