Abstract
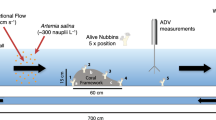
For poikilothermic organisms in aquatic environment, temperature strongly affects metabolism and modulates the extent to which it is mass transfer limited. The effects of temperature on metabolism are translated into organism performance depending on the organism size, morphology, and the flow regime to which they are exposed. Environmental forcing of metabolism is particularly germane to tropical reef corals, because they increasingly are exposed to upward temperature perturbations to which they display variable responses that remain incompletely understood. In this study, we tested the effects of colony size and seawater turbulence on the response of the common Indo-Pacific branching coral, Pocillopora verrucosa, to different seawater temperatures. Using whole-colony calcification as a response variable, 12 tanks (each 150 l) were used in two trials lasting 14 days to contrast the effects of seawater turbulence (two levels) and temperature (25.5 vs 29.5 °C) on colonies varying in size from ~ 4 to 13-cm diameter. Turbulence in the tanks was measured as the root mean square (rms) turbulent flow speed (q rms), which quantified the variation in speed of complex and non-linear flow, regardless of direction. Treatments contrasted low (q rms = 0.63 cm s−1) and high (q rms = 2.07 cm s−1) turbulence that were ecologically relevant for shallow coral reefs. The effects of turbulent flow speed and temperature on calcification (mg day−1) differed among colony sizes: at 25.5 °C, calcification increased with tissue surface area at a slower rate under high, compared to low turbulent flow speeds; at low turbulent flow speeds, calcification increased with tissue surface area more slowly at 29.5 versus 25.5 °C. These results show that the response of branching corals to temperatures depends on colony size and turbulent flow speed. This outcome suggests that spatial heterogeneity in turbulent flow regimes across a coral reef may provide opportunities for branching corals to exploit colony size to modulate susceptibility to rising temperature.



Similar content being viewed by others
References
Ainsworth TD, Heron SF, Ortiz JC, Mumby PJ, Grech A, Ogawa D, Eakin CM, Leggat W (2016) Climate change disables coral bleaching protection on the Great Barrier Reef. Science 352:338–342
Allemand D, Tambutté É, Zoccola D, Tambutté S (2011) Coral calcification: cells to reefs. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, New York, pp 119–150
Atkinson MJ, Bilger RW (1992) Effects of water velocity on phosphate uptake in coral reef-flat communities. Limnol Oceanogr 37:273–279
Baker DM, Freeman CJ, Knowlton N, Thacker RW, Kim K, Fogel ML (2015) Productivity links morphology, symbiont specificity and bleaching in the evolution of Caribbean octocoral symbioses. ISME J 9:2620–2629
Bramanti L, Edmunds PJ (2016) Density-associated recruitment mediates coral population dynamics on a coral reef. Coral Reefs 35:543–553
Brown B, Bythell J (2005) Perspectives on mucus secretion in reef corals. Mar Ecol Prog Ser 296:291–309
Brown BE, Dunne RP (2015) Coral Bleaching: the roles of sea temperature and solar radiation. In: Woodley CM, Downs CA, Bruckner AW, Porter JW, Galloway SB (eds) Diseases of coral. Wiley, Amsterdam, pp 266–283
Burgess SC, Ryan WH, Blackstone NW, Edmunds PJ, Hoogenboom MO, Levitan DR, Wulf JL (2017) Metabolic scaling in modular animals. Invertebrate Biology (in press)
Carpenter RC, Williams SL (2007) Mass transfer limitation of photosynthesis of coral reef algal turfs. Mar Biol 151:435–450
Castillo KD, Ries JB, Bruno JF, Westfield IT (2014) The reef-building coral Siderastrea siderea exhibits parabolic responses to ocean acidification and warming. Proc R Soc Lond B Biol Sci 281:20141856
Chamberlain JA, Graus RR (1975) Water flow and hydromechanical adaptations of branched reef corals. Bull Mar Sci 25:112–125
Chan NCS, Wangpraseurt D, Kühl M, Connolly SR (2016) Flow and coral morphology control coral surface pH: implications for the effects of ocean acidification. Front Mar Sci 3:1–11
Chang S, Elkins C, Alley M, Eaton J, Monismith S (2009) Flow inside a coral colony measured using magnetic resonance velocimetry. Limnol Oceanogr 54:1819–1827
Chang S, Iaccarino G, Ham F, Elkins C, Monismith S (2014) Local shear and mass transfer on individual coral colonies: computations in unidirectional and wave-driven flows. J Geophys Res Ocean 119:2599–2619
Coles SL, Jokiel PL (1977) Effects of temperature on photosynthesis and respiration in hermatypic corals. Mar Biol 43:209–216
Comeau S, Edmunds PJ, Lantz CA, Carpenter RC (2014) Water flow modulates the response of coral reef communities to ocean acidification. Sci Rep 4:6681
Crossland CJ (1987) In situ release of mucus and DOC-lipid from the corals Acropora variabilis and Stylophora pistillata in different light regimes. Coral Reefs 6:35–42
Dennison WC, Barnes DJ (1988) Effect of water motion on coral photosynthesis and calcification. J Exp Mar Biol Ecol 115:67–77
Dickson AG, Sabine CL, Christian JR (2007) Guide to best practices for ocean CO2 measurements. PICES Spec Publ 3:191
Edmunds PJ (2005) The effect of sub-lethal increases in temperature on the growth and population trajectories of three scleractinian corals on the Southern Great Barrier Reef. Oecologia 146:350–364
Edmunds PJ, Burgess SC (2016) Size-dependent physiological responses of the branching coral Pocillopora verrucosa to elevated temperature and pCO2. J Exp Biol 219:3896–3906
Edmunds PJ, Leichter JJ (2016) Spatial scale-dependent vertical zonation of coral reef community structure in French Polynesia. Ecosphere 7:e01342
Edmunds PJ, Leichter JJ, Johnston EC, Tong EJ, Toonen RJ (2016) Ecological and genetic variation in reef-building corals on four Society Islands. Limnol Oceanogr 61:543–557
Falter JL, Atkinson MJ, Coimbra CFM (2005) Effects of surface roughness and oscillatory flow on the dissolution of plaster forms: evidence for nutrient mass transfer to coral reef communities. Limnol Oceanogr 50:246–254
Falter JL, Atkinson MJ, Lowe RJ, Monismith SG, Koseff JR (2007) Effects of nonlocal turbulence on the mass transfer of dissolved species to reef corals. Limnol Oceanogr 52:274–285
Finelli CM, Helmuth BST, Pentcheff ND, Wethey DS (2006) Water flow influences oxygen transport and photosynthetic efficiency in corals. Coral Reefs 25:47–57
Gaines S, Roughgarden J (1985) Larval settlement rate: a leading determinant of structure in an ecological community of the marine intertidal zone. Proc Natl Acad Sci USA 82:3707–3711
Goldenheim WM, Edmunds PJ (2011) Effects of flow and temperature on growth and photophysiology of scleractinian corals in Moorea, French Polynesia. Biol Bull 221:270–279
Hadfield JD (2010) MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Softw 33:1–22
Hadfield JD (2014) MCMCglmm Course notes. pp 140. https://cran.r-project.org/web/packages/MCMCglmm/vignettes/CourseNotes.pdf
Hoogenboom M, Beraud E, Ferrier-Pages C (2010) Relationship between symbiont density and photosynthetic carbon acquisition in the temperate coral Cladocora caespitosa. Coral Reefs 29:21–29
Hughes RN (2005) Lessons in modularity: the evolutionary ecology of colonial invertebrates. Sci Mar 69:169–179
Hughes TP, Kerry JT, Alvarez-Noriega M, Alvarez-Romero J et al (2017) Global warming and recurrent mass bleaching of corals. Nature 543:371–377
Jackson JBC (1979) Morphological strategies of sessile animals. In: Jackson JBC, Buss LW, Cook RE (eds) Population biology and evolution of clonal organisms. Yale University Press, New Haven, pp 499–555
Jimenez IM, Kühl M, Larkum AWD, Ralph PJ (2008) Heat budget and thermal microenvironment of shallow-water corals: do massive corals get warmer than branching corals? Limnol Oceanogr 53:1548–1561
Jokiel PL, Morrissey JI (1986) Influence of size on primary production in the reef coral Pocillopora damicornis and the macroalga Acanthophora spicifera. Mar Biol 91:15–26
Kayal M, Vercelloni J, Lison de Loma T et al (2012) Predator Crown-of-Thorns Starfish (Acanthaster planci) outbreak, mass mortality of corals, and cascading effects on reef fish and benthic communities. PLoS One 7:e47363
Kuffner IB, Hickey TD, Morrison JM (2013) Calcification rates of the massive coral Siderastrea siderea and crustose coralline algae along the Florida Keys (USA) outer-reef tract. Coral Reefs 32:987–997
Kühl M, Cohen Y, Dalsgaard T, Jørgensen BB, Revsbech NP (1995) Microenvironment and photosynthesis of zooxanthellae in scleractinian corals studied with microsensors for O2, pH and light. Mar Ecol Progr Ser 117:159–172
Leichter J of Moorea coral reef LTER (2015) MCR LTER: Coral reef: benthic water temperature, ongoing since 2005. knb-lter-mcr.1035.10 https://doi.org/10.6073/pasta/64651327973d5dfba8ef75f886f7d106
Leichter J, Shellenbarger G, Genovese S, Wing S (1998) Breaking internal waves on a Florida (USA) coral reef: a plankton pump at work? Mar Ecol Progr Ser 166:83–97
Lenihan HS, Hench JL, Holbrook SJ, Schmitt RJ, Potoski M (2015) Hydrodynamics influence coral performance through simultaneous direct and indirect effects. Ecology 96:1540–1549
Lesser MP, Weis VM, Patterson MR, Jokiel PL (1994) Effects of morphology and water motion on carbon delivery and productivity in the reef coral, Pocillopora damicornis (Linnaeus): diffusion barriers, inorganic carbon limitation, and biochemical plasticity. J Exp Mar Biol Ecol 178:153–179
Lowe RJ, Falter JL (2015) Oceanic forcing of coral reefs. Ann Rev Mar Sci 7:43–66
Lowe RJ, Koseff JR, Monismith SG, Falter JL (2005) Oscillatory flow through submerged canopies: 2. Canopy mass transfer. J Geophys Res 110:C10017
Madin JS, Hughes TP, Connolly SR (2012) Calcification, storm damage and population resilience of tabular corals under climate change. PLoS One 7:e46637
Marti-Puig P, Forsman ZH, Haverkort-Yeh RD, Knapp ISS, Maragos JE, Toonen RJ (2014) Extreme phenotypic polymorphism in the coral genus Pocillopora; micro-morphology corresponds to mitochondrial groups, while colony morphology does not. Bull Mar Sci 90:211–231
Mass T, Genin A, Shavit U, Grinstein M, Tchernov D (2010) Flow enhances photosynthesis in marine benthic autotrophs by increasing the efflux of oxygen from the organism to the water. Proc Natl Acad Sci USA 107:2527–2531
Mayer AG (1917) Is death from high temperature due to the accumulation of acid in the tissues? Am J Physiol 44:581–585
McClanahan T, Maina J, Moothien-Pillay R, Baker A (2005) Effects of geography, taxa, water flow, and temperature variation on coral bleaching intensity in Mauritius. Mar Ecol Prog Ser 298:131–142
Monismith SG (2007) Hydrodynamics of coral reefs. Annu Rev Fluid Mech 39:37–55
Nakamura T, van Woesik R (2001) Water-flow rates and passive diffusion partially explain differential survival of corals during the 1998 bleaching event. Mar Ecol Prog Ser 212:301–304
Nakamura T, Yamasaki H, van Woesik R (2003) Water flow facilitates recovery from bleaching in the coral Stylophora pistillata. Mar Ecol Prog Ser 256:287–291
Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL (2011) Projecting coral reef futures under global warming and ocean acidification. Science 333:418–422
Patterson MR (1992a) A mass transfer explanation of metabolic scaling relations in some aquatic invertebrates and algae. Science 255:1421–1423
Patterson MR (1992b) A chemical engineering view of cnidarian symbioses. Integr Comp Biol 32:566–582
Patterson MR, Sebens KP (1989) Forced convection modulates gas exchange in cnidarians. Proc Natl Acad Sci USA 86:8833–8836
Patterson MR, Sebens KP (1991) In situ measurements of flow effects on primary production and dark respiration in reef corals. Limnol Oceanogr 36:936–948
Pratchett MS, Anderson KD, Hoogenboom MO, Widman E, Baird AH, Pandolfi JM, Edmunds PJ, Lough JM (2015) Spatial, temporal and taxonomic variation in coral growth-implications for the structure and function of coral reef ecosystems. Oceanogr Mar Biol Annu Rev 53:215–295
Rahav O, Dubinsky Z, Achituv Y, Falkowski PG (1989) Ammonium metabolism in the zooxanthellate coral, Stylophora pistillata. Proc R Soc Lond B Biol Sci 236:325–337
Reidenbach M, Koseff J, Monismith S (2006) The effects of waves and morphology on mass transfer within branched reef corals. Limnol Oceanogr 51:1134–1141
Rivest EB, Gouhier TC (2015) Complex environmental forcing across the biogeographical range of coral populations. PLoS One 10:e0121742
Rosen BR (1986) Modular growth and form of corals: a matter of metamers. Philos Trans R Soc Lond B Biol Sci 313:115–142
Rosenberg E, Koren O, Reshef L, Efrony R, Zilber-Rosenberg I (2007) The role of microorganisms in coral health, disease and evolution. Nat Rev Microbiol 5:355–362
Schmidt-Nielsen K (1984) Scaling: why is animal size so important?. Cambridge University Press, Cambridge
Schutter M, Crocker J, Paijmans A, Janse M, Osinga R, Verreth AJ, Wijffels RH (2010) The effect of different flow regimes on the growth and metabolic rates of the scleractinian coral Galaxea fascicularis. Coral Reefs 29:737–748
Spencer-Davies P (1989) Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar Biol 101:389–395
Stimson J, Kinzie RA (1991) The temporal pattern and rate of release of zooxanthellae from the reef coral Pocillopora damicornis (Linnaeus) under nitrogen-enrichment and control conditions. J Exp Mar Biol Ecol 153:63–74
Thomas FIM, Atkinson MJ (1997) Ammonium uptake by coral reefs: effects of water velocity and surface roughness on mass transfer. Limnol Oceanogr 42:81–88
Tsounis G, Edmunds PJ (2016) The potential for self-seeding by the coral Pocillopora spp. in Moorea, French Polynesia. PeerJ 4:e2544
Veron JEN (2000) Corals of the World, Australian Institute of Marine Science and CRR Ald Pty Ltd
Vollmer SV, Edmunds PJ (2000) Allometric scaling in small colonies of the scleractinian coral Siderastrea siderea (Ellis and Solander). Biol Bull 199:21–28
Washburn L of Moorea Coral Reef LTER (2016) MCR LTER: Coral reef: ocean currents and biogeochemistry: salinity, temperature and current at CTD and ADCP mooring FOR01 from 2004 ongoing. knb-lter-mcr.30.31 https://doi.org/10.6073/pasta/5f53dda2c4655f8301de82f472f5c10b
Acknowledgements
We thank V. Moriarty and C. Lantz for technical support, D. Sternberg, J. Bergmann, S. Zimmermann, H. Nelson for field assistance, L. Washburn for valuable discOussion of seawater flow on coral reefs and among coral branches, and the staff of the UC Berkeley Richard B. Gump South Pacific Research Station for making our visits to Moorea productive and enjoyable. This is contribution number 259 of the marine biology program of California State University, Northridge.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing or financial interests.
Funding
This study was funded by the US National Science Foundation through the Moorea Coral Reef LTER (OCE 10-26852) and grants for research on ocean acidification (OCE 10-41270, 14-15268).
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Data accessibility
Data in support of this manuscript deposited at the MCR-LTER site: http://mcr.lternet.edu/data/ (https://doi.org/10.6073/pasta/d583e9ea5529c11cbc2c2884fd77ae14).
Additional information
Responsible Editor: L. Mydlarz.
Reviewed by Undisclosed experts.
Rights and permissions
About this article
Cite this article
Edmunds, P.J., Burgess, S.C. Colony size and turbulent flow speed modulate the calcification response of the coral Pocillopora verrucosa to temperature. Mar Biol 165, 13 (2018). https://doi.org/10.1007/s00227-017-3257-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3257-z