Abstract
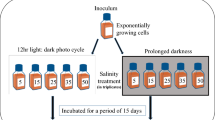
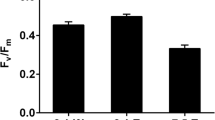
Intertidal rockpools (RPs), forming a ubiquitous component of rocky shores, are biologically rich ecosystems influenced by short-term (hours–days) and long-term (days–seasons) fluctuating environments. So far, studies on RP biology are scarce and received no attention in India. This study elucidates planktonic microalgal composition and photoprotection mechanisms [dynamic photoinhibition, non-photochemical-quenching (NPQ), and photoprotective pigments production)] from the RPs located at high tide (HT), mid tide (MT), and low tide (LT) zones on the rocky shores of Anjuna, Goa (India) facing the Arabian Sea. MT-RPs and LT-RPs were dominated by diatoms and HT-RPs by dinoflagellates due to the blooms of autotrophic benthic dinoflagellates belonging to Amphidinium sensu stricto and Bysmatrum. The detailed microscopic analysis of these dinoflagellates showed morphological and cellular features similar to Amphidinium carterae (known harmful algae of concern) and Bysmatrum caponii. This study reports B. caponii for the first time from India as well as from northern Indian Ocean. The fast-repetition-rate-fluorometer measurements of RP microalgae suggested lower quantum efficiency (F v/F m) and functional absorption cross section for HT-RPs followed by MT-RPs and LT-RPs. The observed differences can thus be attributed to the microalgal composition differences and to differences in experienced irradiance of these communities. Dynamic photoinhibition was more prominent in LT-RPs followed by MT-RPs and HT-RPs. The high accumulation of photoprotective pigments in HT-RPs (due to prolong exposure to solar radiation) could be the reason for the differences. The presence of reduced de-epoxidation state and the mid-day depression in F v/F m coupled with elevated σ PSII confirmed dominance of NPQ of reaction centres in HT-RPs compared to other pools. This study concludes that RP planktonic microalgae are eurythermal, euryhaline, and euryphotic. Concerned with increasing harmful algal bloom events further studies on diverse aspects of RP microalgae (including chemical mediated interactions) needs attention.









Similar content being viewed by others
References
Al-Has A, Noor MN (2011) Identification of marine sand-dwelling dinoflagellates in Dinawan Island, Sabah. Borneo. Science 28:37–45
Almazán-Becerril A, Escobar-Morales S, Rosiles-González G, Valadez F (2015) Benthic-epiphytic dinoflagellates from the northern portion of the Mesoamerican Reef System. Bot Mar 58(2):115–128
Anderson DM, Alpermann TJ, Cembella AD, Collos Y, Masseret E, Montresor M (2012) The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 14:10–35
Babin M, Morel A, Falkowski PG, Claustre H, Bricaud A, Kobler Z (1996) Nutrient and light-dependent variations of the maximum quantum yield of carbon fixation in eutrophic, mesotrophic and oligotrophic systems. Deep Sea Res I 43(8):1241–1272
Baig HS, Saifullah SM, Dar A (2006) Occurrence and toxicity of Amphidinium carterae Hulburt in the North Arabian Sea. Harmful Algae 5(2):133–140
Barlow R, Stuart V, Lutz V, Sessions H, Sathyendranath S, Platt T, Kyewalyanga M, Clementson L, Fukasawa M, Watanabe S, Devred E (2007) Seasonal pigment patterns of surface phytoplankton in the subtropical southern hemisphere. Deep Sea Res I(54):1687–1703
Bauer I, Maranda L, Shimizu Y, Peterson RW, Cornell L, Steiner JR, Clardy J (1994) The structures of Amphidinolide B isomers: strongly cytotoxic macrolides produced by a free-swimming dinoflagellate, Amphidinium sp. J Am Chem Soc 116:2657–2658
Bauer I, Maranda L, Young KA, Shimizu Y, Fairchild C, Cornell L, MacBeth J, Huang S (1995) Isolation and structure of Caribenolide I, a highly potent antitumour macrolide from a culture of the free swimming Caribbean dinoflagellate, Amphidinium sp. S1-36-5. J Org Chem 60:1084–1086
Blackwell JR, Gilmour DJ (1991) Stress tolerance of the tidal pool chlorophyte Chlorococcum submarinum. Brit J Phycol 26:141–147
Brewin RJW, Sathyendranath S, Jackson T, Barlow R, Brotas V, Airs R, Lamont T (2015) Influence of light in the mixed-layer on the parameters of a three-component model of phytoplankton size class. Remote Sens Environ 168:437–450
Brunet C, Johnsen G, Lavaud J, Roy S (2011) Pigments and photoacclimation processes. In: Roy S, Llewellyn CA, Egeland ES, Johnsen G (eds) Phytoplankton pigments, 1st ed, Chapter 11. Cambridge Press University, Cambridge, pp 445–471
Damjanovic A, Ritz T, Schulten K (2000) Excitation transfer in the peridinin-chlorophyll-a-protein of Amphidinium carterae. Biophys J 79:1695–1705
Demmig-Adams B (1990) Carotenoids and photoprotection: a role for the xanthophyll zeaxanthin. Biochim Biophys Acta Bioenerg 1020:1–24
Demmig-Adams B, Adams WWIII (1996) The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci 1:21–26
Dethier MN (1980) Tidepools as refuges: predation and the limits of the harpacticoid copepod Tigriopus californicus (Baker). J Exp Mar Biol Ecol 42:99–111
Dimier C, Corato F, Tramontano F, Brunet C (2007) Photoprotection and xanthophyll-cycle activity in three marine diatoms. J Phycol 43:937–947
Dolapsakis NP, Economou-Amilli A (2009) A new marine species of Amphidinium (Dinophyceae) from Thermaikos Gulf, Greece. Acta Protozool 48:153–170
Dubinsky Z (1992) The functional and optical absorption cross-sections of phytoplankton photosynthesis. In: Falkowskii PG, Woodhead AD (eds) Primary productivity and biogeochemical cycles in the sea. Plenum Press, New York, pp 31–45
Falkowski PG, Dubinsky Z (1981) Light-shade adaptation of Stylophora pistillata, a hermatypic coral from the Gulf of Eilat. Nature 289:172–174
Faust MA (2000) Dinoflagellate associations in a coral reef-mangrove ecosystem: pelican and associated cays, Belize. Atoll Res Bull No. 473
Faust MA, Steidinger KA (1998) Bysmatrum gen. nov. (Dinophyceae) and three new combinations for benthic Scrippsielloid species. Phycologia 37:47–52
Faust MA, Litaker RW, Vandersea MW, Kibler SR, Tester PA (2005) Dinoflagellate diversity and abundance in two Belizean coral-reef mangrove lagoons: a test of Margalef’s Mandala. Atoll Research Bull No. 534
Flø Jørgensen M, Murray S, Daugbjerg N (2004) Amphidinium revisited. I. Redefinition of Amphidinium (Dinophyceae) based on cladistic and molecular phylogenetic analyses. J Phycol 40:351–365
From N, Richardson K, Mousing EA, Jensen PE (2014) Removing the light history signal from normalized variable fluorescence (F v/F m) measurements on marine phytoplankton. Limnol Oceanogr Methods 12(11):776–783
Gorbunov MY, Kolber ZS, Lesser MP, Falkowski PG (2001) Photosynthesis and photoprotection in symbiotic corals. Limnol Oceanogr 46(1):75–85
Gottschling M, Soehner S, Zinssmeister C, John U, Plötner J, Schweikert M, Aligizaki K, Elbrächter M (2012) Delimitation of the Thoracosphaeraceae (Dinophyceae), including the calcareous dinoflagellates, based on large amounts of ribosomal RNA sequence data. Protist 163:15–24
Häggqvist K, Lindholm T (2015) Phytoplankton communities in rock pools on the Åland Islands, SW Finland–environmental variables, functional groups and strategies. Biodiversity 16(1):15–26
Hazeem LJ (2009) Molecular techniques for investigating toxic dinoflagellate species in the western English Channel, UK and in Bahrain coastal waters of the Arabian Gulf. University of Southampton, Faculty of Engineering Science and Mathematics, School of Ocean and Earth Science, Doctoral Thesis, p 209
Hinzmann M (2005) Study on benthic dinoflagellates from the Aveiro lagoon (NW Portugal). Dissertation Thesis
Horiguchi T, Pienaar RN (2000) Validation of Bysmatrum arenicola Horiguchi et Pienaar sp. nov. (Dinophyceae). J Phycol 36:237
Houdai T, Matsuoka S, Murata M, Satake M, Ota S, Oshima Y, Rhodes L (2001) Acetate labelling patterns of dinoflagellate polyketides, amphidinols 2, 3, and 4. Tetrahedron 57:5551–5555
Huggett J, Griffiths CL (1986) Some relationships between elevation, physico–chemical variables and biota of intertidal rock pools. Mar Ecol Prog Ser 29:189–197
Jeong HJ, Kang H, Shim JH, Park JK, Kim JS, Song JY, Choi HJ (2001) Interactions among the toxic dinoflagellate Amphidinium carterae, the heterotrophic dinoflagellate Oxyrrhis marina, and the calanoid copepods Acartia spp. Mar Ecol Prog Ser 218:77–86
Jeong HJ, Jang SH, Kang NS, Yoo YD, Kim MJ, Lee KH, Yoon EY, Potvin E, Hwang YJ, Kim JI, Seong KA (2012) Molecular characterization and morphology of the photosynthetic dinoflagellate Bysmatrum caponii from two solar Saltons in Western Korea. Ocean Sci J 47(1):1–18
Johnson MP (2000) Physical control of plankton population abundance and dynamics in intertidal rock pools. Hydrobiologia 440:145–152
Jonsson PR (1994) Tidal rhythm of cyst formation in the rock pool ciliate Strombidium oculatum Gruber (Ciliophora, Oligotrichida): a description of the functional biology and an analysis of the tidal synchronization of encystment. J Exp Mar Biol Ecol 175:77–103
Jung SW, Joo HM, Park JS, Lee JH (2010) Development of a rapid and effective method for preparing delicate dinoflagellates for scanning electron microscopy. J Appl Phycol 22(3):313–317
Kleima-Foske J, Hofmann E, Gobets B, van Stokkum IHM, van Grondelle R, Diederichs K, van Amerongen H (2000a) Forster excitation energy transfer in peridinin-chlorophyll-a-protein. Biophys J 78:344–353
Kleima-Foske J, Hofmann E, Wendling M, Hofmann K, Peterman EJG, van Grondelle R, van Amerongen H (2000b) Peridinin chlorophyll a protein: relating structure and steady-state spectroscopy. Biochemistry 39:5184–5195
Kobayashi J, Shigemori H, Ishibashi M, Yamasu T, Hirota H, Sasaki T (1991) Amphidinolides G and H: new potent cytotoxic macrolides from the cultured symbiotic dinoflagellate Amphidinium sp. J Org Chem 56:5221–5224
Kolber Z, Zehr J, Falkowskii PG (1988) Effects of growth irradiance and nitrogen limitation on photosynthetic energy conversion in photosystem II. Plant Physiol 88:923–929
Kolber Z, Wyman KD, Falkowskii PG (1990) Natural variability in photosynthetic energy conversion efficiency: a study in the Gulf of Maine. Limnol Oceanogr 35:72–79
Krakhmalny A, Bryantseva Y, Velikova V, Sergeeva O, Skuratova K, Dereziuk N (2012) Black Sea Dinoflagellata (history of the research and current biodiversity). Turk J Fish Aquat Sci 12:539–546
Kubota T, Iinuma Y, Kobayashi J (2006) Cloning of polyketide synthase genes from amphidinolide-producing, dinoflagellate Amphidinium sp. Biol Pharm Bull 29:1314–1318
Lee JJ, Shpigel M, Freeman S, Zmora O, McLeod S, Bowen S, Pearson M, Szostek A (2003) Physiological ecology and possible control strategy of a toxic marine dinoflagellate, Amphidinium sp., from the benthos of a mariculture pond. Aquaculture 217:351–371
Ley AC, Mauzerall D (1982) Absolute absorption cross-sections for photosystem II and the minimum quantum requirement for photosynthesis on Chlorella vulgaris. Biochim Biophys Acta 680:95–106
Limoges A, Mertens KN, Ruíz-Fernández AC, de Vernal A (2015) First report of fossilized cysts produced by the benthicBysmatrum subsalsum(Dinophyceae) from a shallow Mexican lagoon in the Gulf of Mexico. J Phycol 51(1):211–215
Mandal SK, Singh RP, Patel V (2011) Isolation and characterization of exopolysaccharide secreted by a toxic dinoflagellate, Amphidinium carterae Hulburt 1957 and its probable role in harmful algal blooms (HABs). Microb Ecol 62:518–527
Martins GM, Hawkins SJ, Thompson RC, Jenkins SR (2007) Community structure and functioning in intertidal rock pools: effects of pool size and shore height at different successional stages. Mar Ecol Prog Ser 329:43–55
Mauzerall D, Green-Baum NL (1989) The absolute size of a photosynthetic unit. Biochim Biophys Acta 974:119–140
Metaxas A, Scheibling RE (1993) Community structure and organization of tidepools. Mar Ecol Prog Ser 98:187–198
Mohammad-Noor N, Daugbjerg N, Moestrup Ø, Anton A (2007) Marine epibenthic dinoflagellates from Malaysia—a study of live cultures and preserved samples based on light and scanning electron microscopy. Nordic J Bot 24(6):629–690
Murray S, Jørgensen MF, Daugbjerg N, Rhodes L (2004) Amphidinium revisited. ii. Resolving species boundaries in the Amphidinium operculatum species complex (dinophyceae), including the descriptions of Amphidinium trulla sp. Nov. and Amphidinium gibbosum. Comb. Nov. J Phycol 40:366–382
Murray S, Hoppenrath M, Larsen J, Patterson DJ (2006) Bysmatrum teressp. nov., a new sand-dwelling dinoflagellate from north-western Australia. Phycologia 45(2):161–167
Murray SA, Garby T, Hoppenrath M, Neilan BA (2012) Genetic diversity, morphological uniformity and polyketide production in dinoflagellates (Amphidinium, Dinoflagellata). PLoS One 7(6):e38253. doi:10.1371/journal.pone.0038253
Murray SA, Kohli GS, Farrell H, Spiers ZB, Place AR, Dorantes-Aranda JJ, Ruszczyk J (2015) A fish kill associated with a bloom of Amphidinium carterae in a coastal lagoon in Sydney, Australia. Harmful Algae 49:19–28
Okolodkov YB, Merino-Virgilio FC, Aké-Castillo JA, Aguilar-Trujillo AC, Espinosa-Matías S, Herrera-Silveira AJ (2014) Seasonal Changes in epiphytic dinoflagellate assemblages near the northern coast of the Yucatan Peninsula, Gulf of Mexico. Act Bot Mex 107:121–151
Olaizola M, La Roche J, Kolber Z, Falkowski PG (1994) Non-photochemical fluorescence quenching and the diadinoxanthin cycle in a marine diatom. Photosynth Res 41(2):357–370
Parsons ML, Preskitt LB (2007) A survey of epiphytic dinoflagellates from the coastal waters of the island of Hawaii. Harmful Algae 6(5):658–669
Patil JS, Anil AC (2015) Effect of monsoonal perturbations on the occurrence of phytoplankton blooms in a tropical bay. Mar Ecol Prog Ser 530:77–92
Paul GP, Matsumori N, Konoki K, Sasaki M, Murata M, Tachibana K (1996) Structure of Amphidinol 3 and its cholesterol-dependent membrane perturbation: a strong antifungal metabolite produced by the dinoflagellate, Amphidinium klebsii. In: Yasumoto T, Oshima Y, Fukuyo Y (eds) Harmful and toxic algal blooms. UNESCO, Paris, pp 503–506
Roy R, Chitari R, Kulkarni V, Krishna MS, Sarma VVSS, Anil AC (2015) CHEMTAX-derived phytoplankton community structure associated with temperature fronts in the northeastern Arabian Sea. J Mar Syst 144:81–91
Ruivo MPM (2010) Characterization by HPLC of phytoplankton and microphytobenthos photosynthetic pigments. Ph. D. Thesis (Mestrado em Ecologia Marinha), p 101
Saburova M, Al-Yamani F, Polikarpov I (2009) Biodiversity of free-living flagellates in Kuwait’s intertidal sediments. In: Krupp F, Musselman LJ, Kotb MMA, Weidig I (Eds) Environment, biodiversity and conservation in the middle east. Proceedings of the First Middle Eastern Biodiversity Congress, Aqaba, Jordan, 20–23 October 2008. BioRisk 3, pp 97–110
Sanil Kumar V, Pathak KC, Pednekar P, Raju NSN, Gowthaman R (2006) Coastal processes along the Indian coastline. Curr Sci 91(4):530–536
Satake M, Murata M, Yasumoto T, Fujita T, Naoki H (1991) Amphidinol, a polyhydroxypolyene antifungal agent with an unprecedented structure, from a marine dinoflagellate, Amphidinium klebsii. J Am Chem Soc 113:9859–9861
Satta CT, Anglès S, Lugliè A, Guillén J, Sechi N, Camp J, Garcés E (2013) Studies on dinoflagellate cyst assemblages in two estuarine Mediterranean bays: a useful tool for the discovery and mapping of harmful algal species. Harmful Algae 24:65–79
Satta CT, Anglès S, Garcés E, Sechi N, Pulina S, Padedda BM, Stacca D, Lugliè A (2014) Dinoflagellate cyst assemblages in surface sediments from three shallow Mediterranean lagoons (Sardinia, North Western Mediterranean Sea). Estua Coast 37(3):646–663
Shah MMR, Reimer JD, Horiguchi T, Suda S (2010) Diversity of dinoflagellate blooms in reef flat tide pools at Okinawa, Japan. Galaxea J Coral Reef Stud 12(1):49–49
Shahi N, Godhe N, Mallik SK, Harnstrom K, Nayak BB (2015) The relationship between variation of phytoplankton species composition and physic chemical parameters in northern coastal waters of Mumbai, India. Ind J Geo Mar Sci 44(5)
Ten-Hage L, Quod JP, Turquet J, Couté A (2001) Bysmatrum granulosum sp. Nov., a new benthic dinoflagellate from the Southwestern Indian ocean. Eur J Phycol 36(2):129–135
Ten Lohuis MR, Miller DJ (1998) Genetic transformation of dinoflagellates (Amphidinium and Symbiodinium): expression of GUS in microalgae using heterologous promoter constructs. Plant J 13:427–435
Therriault TW, Kolasa J (2001) Desiccation frequency reduces species diversity and predictability of community structure in coastal rock pools. Israel J Zool 47:477–489
Underwood AJ, Skilleter GA (1996) Effects of patch size on the structure of assemblages in rock pools. J Exp Mar Biol Ecol 197:63–90
Van Heukelem L (2002) HPLC phytoplankton pigments: sampling, laboratory methods, and quality assurance procedures. In: Mueller J, Fargion G (eds) Ocean optics protocols for satellite ocean color sensor, Revision 3, volume 2, Chap. 16, NASA Technical Memorandum 2002–2004, vol. 2, pp 258–268
Van de Poll WH, Alderkamp AC, Janknegt PJ, Roggeveld J, Buma AGJ (2006) Photoacclimation modulates excessive photosynthetically active and ultraviolet radiation effects in a temperate and Antarctic marine diatom. Limnol Oceanogr 51:1239–1248
van de Poll WH, Buma AGJ (2009) Does ultraviolet radiation affect the xanthophyll cycle in marine phytoplankton? Photochem Photobiol Sci 8(9):1295
Vassiliev IR, Prasil O, Wyman KD, Kolber Z, Hanson AK, Prentice JE, Falkowski PG (1994) Inhibition of PSII photochemistry by PAR and UV radiation in natural phytoplankton communities. Photosynth Res 42:51–64
Vijith V, Sundar D, Shetye SR (2009) Time-dependence of salinity in monsoonal estuaries. Estuar Coast Shelf Sci 85:601–608
Villafañe V, Janknegt PJ, de Graaff M, Visser RJW, Van de Poll WH, Buma AGJ, Helbling EW (2008) UVR-induced photoinhibition of summer marine phytoplankton communities from Patagonia. Mar Biol 154:1021–1029
Yuki K, Fukuyo Y (1992) Alexandrium satoanum sp. nov. (Dinophyceae) from Matoya Bay, central Japan. J Phycol 28:395–399
Acknowledgements
We are grateful to the Director of CSIR-National Institute of Oceanography for his support and encouragement. We thank Drs. NL Thakur and D Desai for co-ordinating intertidal rock pool experiments under the Ocean Findere project and the project staff, who were involved in the experiments, for their help during sampling. We are also thankful to the two anonymous reviewers for their suggestions in improving the manuscript. We also thank Mr. Areef Sardar with scanning electron microscopy (SEM). This is an NIO contribution No. 6017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by the Council of Scientific and Industrial Research (CSIR) funded project Ocean Finder PSC 0105.
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Additional information
Responsible Editor: K. Bischof.
Reviewed by Undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patil, J.S., Rodrigues, R.V., Paul, P. et al. Benthic dinoflagellate blooms in tropical intertidal rock pools: elucidation of photoprotection mechanisms. Mar Biol 164, 89 (2017). https://doi.org/10.1007/s00227-017-3123-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3123-z