Abstract
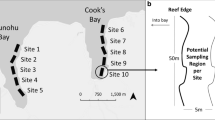
Despite the massive expansion of the invasive corals Tubastraea spp. in the Tropical Western Atlantic, some sponge species may outcompete them on a local scale. The aims of the present study were: (1) to describe the spatiotemporal dynamics of the benthic community and (2) to assess the interactions between marine sponges and invasive Tubastraea corals. Communities were monitored at four locations and four times (2013–2015) in Ilha Grande Bay, southeastern Brazil. The percent cover of the dominant taxa in the benthic communities was calculated and all interactions among native sponges and Tubastraea spp. corals counted within photoquadrats. These in situ observations were used to assess four categories of interaction types. We did not find statistical differences in the benthic communities among locations and times. Turf forming algae and Palythoa caribaeorum represented 60–70% of the benthic community. The number and types of interactions between sponges and corals differed significantly among locations. The most common interaction was contact without dominance. Iotrochota arenosa and Scopalina ruetzleri were the most common sponge species competing with Tubastraea spp. Furthermore, Desmapsamma anchorata and I. arenosa were the main sponge species able to occasionally kill the invasive corals by overgrowth. However, the slow rate of overgrowth by sponges was not able to prevent the fast expansion of the non-indigenous corals. Hence, population studies on native and alien species may help predict the effects of biological invasion on local biodiversity.




Similar content being viewed by others
References
Aerts LAM (1998) Sponge/coral interactions in Caribbean reefs: analysis of overgrowth patterns in relation to species identity and cover. Mar Ecol Prog Ser 175:241–249
Aerts LAM, van Soest R (1997) Quantification of sponge/coral interactions in a physically stressed reef community, NE Colombia. Mar Ecol Prog Ser 148:125–134
Agrawal AA, Ackerly DD, Adler F et al (2007) Filling key gaps in population and community ecology. Front Ecol Environ 5:145–152. doi:10.1890/1540-9295
Albins MA (2013) Effects of invasive Pacific red lionfish Pterois volitans versus a native predator on Bahamian coral-reef fish communities. Biol Invasions 15:29–43. doi:10.1007/s10530-012-0266-1
Bakus GJ (2007) Quantitative analysis of marine biological communities: field biology and environment. Wiley, USA
Bastidas C, Bone D (1996) Competitive strategies between Palythoa caribaeorum and Zoanthus sociatus (Cnidaria: Anthozoa) at a reef flat environment in Venezuela. Bull Mar Sci 59:543–555
Bell JJ (2008) The functional roles of marine sponges. Estuar Coast Shelf Sci 79:341–353
Bell JJ, Barnes DKA (2003) The importance of competitor identity, morphology and ranking methodology to outcomes in interference competition between sponges. Mar Biol 143:415–426. doi:10.1007/s00227-003-1081-0
Bottollier-Curtet M, Planty-Tabacchi AM, Tabacchi E (2013) Competition between young exotic invasive and native dominant plant species: implications for invasions within riparian areas. J Veg Sci 24:1033–1042
Buss L, Jackson JBC (1979) Competitive networks: nontransitive competitive relationships in cryptic coral reef environments. Am Nat 113:223–234
Carpenter RC (1986) Partitioning herbivory and its effects on coral reef algal communities. Ecol Monogr 56:345–364. doi:10.2307/1942551
Castello-Branco C, Menegola C (2014) Sponges from the Ilha Grande Bay, Rio de Janeiro State: two new records for Brazilian south-east region. Mar Biodivers Rec 7:e21. doi:10.1017/S1755267214000116
Castro CB, Pires DO (2001) Brazilian coral reefs: what we already know and what is still missing. Bull Mar Sci 69:357–371
Castro CB, Echeverría CA, Pires DDO et al (1999) Distribuição do bentos (Cnidaria e Echinodermata) em costões rochosos da Baía de Ilha Grande, Rio de Janeiro, Brasil. Oecologia Bras 7:179–194
Creed JC, Paula AF (2007) Substratum preference during recruitment of two invasive alien corals onto shallow-subtidal tropical rocky shores. Mar Ecol Prog Ser 330:101–111
Creed JC, Pires D, Figueiredo M (2007) Biodiversidade marinha da Baía de Ilha Grande. Brasília. Ministério do Meio Ambiente, Brasília, Série Biodiversidade 23, pp 417
Creed JC, Fenner D, Sammarco P et al (2016) The invasion of the azooxanthellate coral Tubastraea (Scleractinea: Dendrophylliidae) throughout the world: history, pathways and vectors. Biol Invasions 19:283–305. doi:10.1007/s10530-016-1279-y
Da Silva AG, De Paula AF, Fleury BG et al (2014) Eleven years of range expansion of two invasive corals (Tubastraea coccinea and Tubastraea tagusensis) through the southwest Atlantic (Brazil). Estuar Coast Shelf Sci 141:9–16. doi:10.1016/j.ecss.2014.01.013
De Voogd NJ, Becking LE, Hoeksema BW et al (2004) Sponge interactions with spatial competitors in the Spermonde Archipelago. Boll di Mus e Ist di Biol dell’Universita di Genova 68:253–261
de Goeij JM, van Oevelen D, Vermeij MJA et al (2013) Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science 342:108–110. doi:10.1126/science.1241981
Diaz C, Rützler K (2001) Sponges: an essential component of Caribbean coral reefs. Bull Mar Sci 69:535–546
Duff J, Hay M (2001) The ecology and evolution of marine consumer-prey interactions. In: Marine community ecology. Sunderland, pp 131–157
Fenner D (2001) Biogeography of three Caribbean corals (Scleractinia) and the invasion of Tubastraea coccinea into the Gulf of Mexico. Bull Mar Sci 69:1175–1189
Fenner D, Banks K (2004) Orange cup coral Tubastraea coccinea invades Florida and the Flower Garden Banks, Northwestern Gulf of Mexico. Coral Reefs 23:505–507. doi:10.1007/s00338-004-0422-x
Fleury BG, Lages BG, Barbosa JP, Kaiser CR, Pinto AC (2008) New hemiketal steroid from the introduced soft coral Chromonephthea braziliensis is a chemical defense against predatory fishes. J Chem Ecol 34:987–993
González-Rivero M, Yakob L, Mumby PJ (2011) The role of sponge competition on coral reef alternative steady states. Ecol Model 222:1847–1853. doi:10.1016/j.ecolmodel.2011.03.020
Green SJ, Akins JL, Maljković A et al (2012) Invasive lionfish drive Atlantic coral reef fish declines. PlosOne 7(3):e32596. doi:10.1371/journal.pone.0032596
Hajdu E, Peixinho S, Fernandez JC (2011) Esponjas marinhas da Bahia: guia de campo e laboratório. Rio de Janeiro, Museu Nacional
Hennessey SM, Sammarco PW (2014) Competition for space in two invasive Indo-Pacific corals-Tubastraea micranthus and Tubastraea coccinea: laboratory experimentation. J Exp Mar Bio Ecol 459:144–150. doi:10.1016/j.jembe.2014.05.021
Ikeda Y, Godoi S, Cacciari P (1989) Um estudo de séries temporais de corrente na Baía de Ilha Grande. In: RelInt Inst Oceanogr USP, pp 1–24
Jackson JB, Buss L (1975) Alleopathy and spatial competition among coral reef invertebrates. Proc Natl Acad Sci USA 72:5160–5163. doi:10.1073/pnas.72.12.5160
Lages BG, Fleury BG, Ferreira CEL et al (2006) Chemical defense of an exotic coral as invasion strategy. J Exp Mar Biol Ecol 328:127–135. doi:10.1016/j.jembe.2005.07.011
Lages BG, Fleury BG, Pinto AC et al (2010) Chemical defenses against generalist fish predators and fouling organisms in two invasive ahermatypic corals in the genus Tubastraea. Mar Ecol 31:473–482. doi:10.1111/j.1439-0485.2010.00376.x
Lages BG, Fleury BG, Menegola C et al (2011) Change in tropical rocky shore communities due to an alien coral invasion. Mar Ecol Prog Ser 438:85–96. doi:10.3354/meps09290
Lages BG, Fleury BG, Hovell AMC et al (2012) Proximity to competitors changes secondary metabolites of non-indigenous cup corals, Tubastraea spp., in the southwest Atlantic. Mar Biol 159:1551–1559. doi:10.1007/s00227-012-1941-6
Lages BG, Fleury BG, Creed JC (2015) A review of the ecological role of chemical defenses in facilitating biological invasion by marine benthic organisms. In: Atta-ur-Rahman (ed) Studies in natural products chemistry. Elsevier, Amsterdam, pp 1–26
Levine JM, Antonio CMD (1999) Elton revisited: a review of evidence linking diversity and invasibility. Oikos 87:15–26
Loh TL, Pawlik JR (2014) Chemical defenses and resource trade-offs structure sponge communities on Caribbean coral reefs. Proc Natl Acad Sci USA 111:4151–4156. doi:10.1073/pnas.1321626111
Luter HM, Duckworth AR, Syms C (2007) Cytotoxic and anti-microbial activity of the sponge Iotrochota sp. as a function of size and spatial competitors. Mar Biol Res 3:312–318. doi:10.1080/17451000701635102
Magurran A (1988) Ecological diversity and its measurements. Princeton, New Jersey
Mantelatto MC, Fleury BG, Menegola C et al (2013) Cost–benefit of different methods for monitoring invasive corals on tropical rocky reefs in the southwest Atlantic. J Exp Mar Bio Ecol 449:129–134. doi:10.1016/j.jembe.2013.09.009
Mantelatto MC, Vidon LF, Silveira RB et al (2016) Host species of the non-indigenous brittle star Ophiothela mirabilis (Echinodermata: Ophiuroidea): an invasive generalist in Brazil? Mar Biodivers Rec 9:8. doi:10.1186/s41200-016-0013-x
McLean EL, Yoshioka PM (2007) Associations and interactions between gorgonians and sponges. In: Custódio MR, Lôbo-Hajdu G, Hajdu E, Muricy G (eds) Porifera research biodiversity, innovation and sustainability. Museu Nacional, Rio de Janeiro, pp 443–448
McLean EL, Rützler K, Pooler PS (2015) Competing for space: factors that lead to sponge overgrowth when interacting with octocoral. Open J Mar Sci 5:64–80. doi:10.4236/ojms.2015.51007
Meurer BC, Lages NS, Pereira OA et al (2010) First record of native species of sponge overgrowing invasive corals Tubastraea coccinea and Tubastraea tagusensis in Brazil. Mar Biodivers Rec 3:e62. doi:10.1017/S1755267210000527
Miranda RJ, Cruz ICS, Barros F (2016) Effects of the alien coral Tubastraea tagusensis on native coral assemblages in a southwestern Atlantic coral reef. Mar Biol 163:45. doi:10.1007/s00227-016-2819-9
Moreira PL, Ribeiro FV, Creed JC (2014) Control of invasive marine invertebrates: an experimental evaluation of the use of low salinity for managing pest corals (Tubastraea spp.). Biofouling 30:639–650. doi:10.1080/08927014.2014.906583
Muricy G, Hajdu E (2006) Porifera brasilis-guia de identificação das esponjas marinhas mais comuns do sudeste do Brasil. Rio de Janeiro. Museu Nacional.
Olenin S, Minchin D, Daunys D (2007) Assessment of biopollution in aquatic ecosystems. Mar Pollut Bull 55:379–394. doi:10.1016/j.marpolbul.2007.01.010
Paula A, Creed JC (2005) Spatial distribution and abundance of nonindigenous coral genus Tubastraea (Cnidaria, Scleractinia) around Ilha Grande, Brazil. Braz J Biol 65:661–673. doi:10.1590/S1519-69842005000400014
Pérez CD, Vila-Nova DA, Santos AM (2005) Associated community with the zoanthid Palythoa caribaeorum (Duchassaing & Michelotti, 1860) (Cnidaria, Anthozoa) from littoral of Pernambuco, Brazil. Hydrobiologia 548:207–215. doi:10.1007/s10750-005-5441-2
Sammarco PW, Atchison AD, Boland GS et al (2012) Geographic expansion of hermatypic and ahermatypic corals in the Gulf of Mexico, and implications for dispersal and recruitment. J Exp Mar Bio Ecol 436–437:36–49. doi:10.1016/j.jembe.2012.08.009
Sampaio CLS, Miranda RJ, Maia-Nogueira R et al (2012) New occurrences of the nonindigenous orange cup corals Tubastraea coccinea and T. tagusensis (Scleractinia: Dendrophylliidae) in Southwestern Atlantic. Check List 8:528–530
Santos LAH, Ribeiro FV, Creed JC (2013) Antagonism between invasive pest corals Tubastraea spp. and the native reef-builder Mussismilia hispida in the southwest Atlantic. J Exp Mar Bio Ecol 449:69–76. doi:10.1016/j.jembe.2013.08.017
Sebens KP, Miles JS (1988) Sweeper tentacles in a gorgonian octocoral: morphological modifications for interference competition. Biol Bull 175:378–387. doi:10.2307/1541729
Singh A, Thakur NL (2016) Significance of investigating allelopathic interactions of marine organisms in the discovery and development of cytotoxic compounds. Chem Biol Interact 243:135–147
Stachowicz JJ (2001) Mutualism, facilitation, and the structure of ecological communities. Bioscience 51:235–246
Sullivan B, Faulkner DJ, Webb L (1983) Siphonodictidine, a metabolite of the burrowing sponge Siphonodictyon sp. that inhibits coral growth. Science 221:1175–1176
Thacker RW, Becerro MA, Lumbang WA et al (1998) Allelopathic interactions between sponges on a tropical reef. Ecology 79:1740–1750. doi:10.1890/0012-9658
Tilman D (1999) The ecological consequences of changes in biodiversity: a search for general principles. Ecology 80:1455–1474
Vitousek PM, Mooney HA, Lubchenco J et al (1997) Human domination of Earth’s ecosystems. Science 277:494–499
Witman J, Dayton P (2001) Rock subtidal communities. In: Bertness M, Gaines S, Hay M (eds) Marine community ecology, Sinauer Associates, Sunderland, pp 339–366
Wulff JL (2006) Resistance vs recovery: morphological strategies of coral reef sponges. Funct Ecol 20:699–708. doi:10.1111/j.1365-2435.2006.01143.x
Zalba S, Ziller S (2007) Manejo adaptativo de espécies exóticas invasoras: colocando a teoria em prática. Nat e Conserv 5:16–22
Zar JH (1996) Biostatistical analysis. Prentice-Hall, Eryelwood Cliffs, NJ, pp 663
Acknowledgements
The authors express their gratitude to Dr. Eduardo Hajdu (Museu Nacional/UFRJ) for his assistance in the field and sponge identification. We also thank the help of the infrastructure from the Laboratório de Ecologia Marinha Bêntica/UERJ and some students and researchers who contributed to the present study, through assistance in the field and lab, such as Fabrine Costa, Mariana Aguiar, Larissa Marques, and Fernanda França; Dr. Vinícius Lima for help in statistical analysis (Universidade do Estado do Rio de Janeiro), and Diana Ugalde (Universidad Nacional Autónoma de México) for field assistance. We acknowledgments Aurélio B.B. Ferreira for help with the manuscript. Also we thank Dra. Sônia Santos for help and the staff from the Centro de Estudo Ambientais e Desenvolvimento Sustentável-CEADS/UERJ for the use of facilities. AGS and HFMF gratefully acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), which granted their Master scholarships. The authors also thank editors and anonymous reviewers for their helpful comments on an earlier version of this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro/FAPERJ (Grant No. E-26/110.353/2014), CAPES-Ciências do Mar (Grant No. 1137/2010), and Programa de Incentivo à Produção Científica, Técnica e Artística (UERJ).
Conflict of interest
All authors declare they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: D. Gochfeld.
Reviewed by D. Fenner and an undisclosed expert.
This article is part of the Topical Collection on Invasive Species.
A. G. Silva and H. F. M. Fortunato have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Silva, A.G., Fortunato, H.F.M., Lôbo-Hajdu, G. et al. Response of native marine sponges to invasive Tubastraea corals: a case study. Mar Biol 164, 78 (2017). https://doi.org/10.1007/s00227-017-3112-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3112-2